 |
Chondroitin sulfate (CS) and dermatan sulfate (DS) chains are representative sulfated glycosaminoglycan (GAG) polysaccharides that are expressed as proteoglycans (PGs), where at least one GAG chain is covalently linked to various protein cores. CSPGs and DSPGs are ubiquitously distributed on cell surfaces and within extra/pericellular matrices, which provide functional microenvironments to regulate diverse cellular processes. These divergent functions of CSPGs and DSPGs are exerted largely through their GAG moieties with structural heterogeneity, which are created by collaborative and/or competitive actions of anabolic enzymes for CS/DS biosynthesis (1, 2). CS backbone consists of a simple, linear polysaccharide, chondroitin (Chn), comprising repeating disaccharide units of glucuronic acid (GlcA) and N-acetylgalactosamine (GalNAc). DS corresponds to a stereoisomer for CS, with varying proportions of iduronic acid (IdoA) in place of GlcA in species- and tissue-dependent manners. These GAGs often exist as CS/DS hybrid chains (1).
The assembly of the CS backbone is initiated by the enzymatic action of GalNAc transferase I (GalNAcT-I) that catalyzes the first GalNAc transfer to the non-reducing terminal GlcA residue in the characteristic tetrasaccharide, GlcA-Gal-Gal-Xyl, which is a linker between CS/DS chains and the corresponding protein cores (see “Biosynthesis of the common tetrasaccharide sequence in the glycosaminoglycan-protein linker region” of this series). The repetitive disaccharide region of the CS backbone is generated by the alternate additions of GlcA and GalNAc residues through the actions of GlcA transferase II (GlcAT-II) and GalNAc transferase II (GalNAcT-II), respectively. These two enzymatic activities are exerted through the combinatorial actions of six homologous glycosyltransferases: Chn synthases (ChSy)-1, -2, and -3, Chn polymerizing factor (ChPF), Chn GalNAc transferases (ChGn)-1, and -2 (1, 2). Their respective gene symbols and aliases are summarized in Table 1. Any pairwise combinations of the former four enzymes, ChSy-1, ChSy-2, ChSy-3, and ChPF, can facilitate Chn polymerization in vitro, indicating their potential role as Chn polymerases (Fig. 1). In contrast, the latter two enzymes, ChGn-1 and ChGn-2, do not contribute to Chn polymerization, but rather fine-tune the CS abundance by regulating the chain length and/or the number of Chn/CS GAGs in cooperation with Chn 4-O-sulfotransferases (C4STs) described below (Fig. 1).
The precursor Chn backbone is maturated by further modifications such as sulfation and uronate epimerization. On the basis of substrate preferences of CS-specific sulfotransferases, the sulfation of Chn backbone can be classified into the 4-O-sulfation and 6-O-sulfation pathways (1-3). As an initial step in the biosynthetic scheme, a non-sulfated disaccharide O unit (GlcA-GalNAc) serves as a common acceptor substrate for two classes of enzymes, C4STs and Chn 6-O-sulfotransferase (C6ST), catalyzing the formation of monosulfated disaccharide A [GlcA-GalNAc(4-O-sulfate)] and C [GlcA-GalNAc(6-O-sulfate)] units, respectively (Fig. 1). The subsequent sulfation of the A and C units can also occur via GalNAc 4-sulfate 6-O-sulfotransferase (GalNAc4S-6ST) and uronyl 2-O-sulfotransferase (UST), forming disulfated disaccharide E [GlcA-GalNAc(4,6-O-disulfate)] and D [GlcA(2-O-sulfate)-GalNAc(4-O-sulfate)] units, respectively (Table 1 and Fig. 1). Interestingly, recent evidence demonstrated that the selective tropism for the two initial sulfation pathways can also be regulated by FAM20B and FAM20C, both of which catalyze phosphorylation of xylose residue in the tetrasaccharide liker (see “Biosynthesis of the common tetrasaccharide sequence in the glycosaminoglycan-protein linker region” of this series) (4, 5).
The GlcA residues in the Chn polymer can be epimerized to IdoA by two GlcA C5-epimerases, DS-epi1 and DS-epi2 (Table 1 and Fig. 1). This reaction initiates the conversion of Chn into its stereoisomer, DS, that is defined by the presence of IdoA-containing disaccharide units, a precursor intermediate iO [IdoA-GalNAc], and its sulfated derivatives, iA [IdoA-GalNAc(4-O-sulfate)], iB [IdoA(2-O-sulfate)-GalNAc(4-O-sulfate)], and iE [IdoA-GalNAc(4,6-O-disulfate)] (1-3). Since the DS-epi-mediated uronate epimerization is reversible, a dermatan-specific 4-O-sufation of iO unit by dermatan 4-O-sulfotransferase (D4ST) that leads to form iA unit appears essential to protect L-ido configuration of newly formed IdoA from back epimerization into GlcA. The further elaboration of iB and iE units are achieved by sulfation of iA unit through the actions of UST and GalNAc4S-6ST, respectively (Fig. 1).
The resultant CS/DS polymers show diverse structures with a variety of combinations of the characteristic disaccharide units, O, A, C, D, E, iA, iB, and iE (Fig. 1). This structural variation enables CS/DS chains to acquire a multifunctional nature.
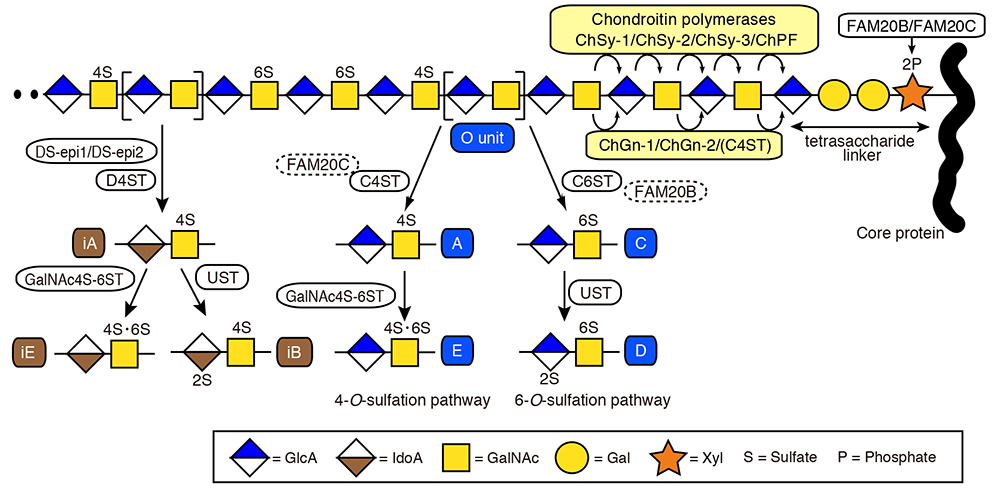
Fig. 1 Biosynthetic pathways for CS/DS chains
Gal and Xyl denote galactose and xylose, respectively. 2S, 4S, 6S, and 2P represent the 2-O-, 4-O-, 6-O-sulfate, and 2-O-phosphate group, respectively.
|
Table 1. CS/DS biosynthetic enzymes in human
| Enzymes |
Abbreviation |
Gene symbol |
Aliases |
Chondroitin synthase |
ChSy-1 |
CHSY1 |
ー |
ChSy-2 |
CHSY2 |
CSS3 |
ChSy-3 |
CHSY3 |
CHPF2, CSGlcA-T |
Chondroitin polymerizing factor |
ChPF |
CHPF |
CSS2 |
Chondroitin GalNAc transferase |
ChGn-1 |
CSGALNACT1 |
ー |
ChGn-2 |
CSGALNACT2 |
ー |
Chondroitin 4-O-sulfotransferase |
C4ST-1 |
C4ST1 |
CHST11 |
C4ST-2 |
C4ST2 |
CHST12 |
C4ST-3 |
C4ST3 |
CHST13 |
Dermatan 4-O-sulfotransferase |
D4ST-1 |
D4ST1 |
CHST14 |
Chondroitin 6-O-sulfotransferase |
C6ST-1 |
C6ST1 |
CHST3 |
Uronyl 2-O-sulfotransferase |
UST |
UST |
ー |
GalNAc 4-sulfate 6-O-sulfotransferase |
GalNAc4S-6ST |
GALNAC4S6ST |
CHST15, BRAG |
GlcA C5 epimerase |
DS-epi1 |
DSE |
SART2 |
DS-epi2 |
DSEL |
C18orf4 |
Tadahisa Mikami & Hiroshi Kitagawa
(Laboratory of Biochemistry, Kobe Pharmaceutical University)
| References |
| (1) |
Mikami T, Kitagawa H: Biosynthesis and function of chondroitin sulfate. Biochim. Biophys. Acta 1830, 4719-4733, 2013 |
| (2) |
Kitagawa H: Using sugar remodeling to study chondroitin sulfate function. Biol. Pharm. Bull. 37, 1705-1712, 2014 |
| (3) |
Kusche-Gullberg M, Kjellén L: Sulfotransferases in glycosaminoglycan biosynthesis. Curr. Opin. Struct. Biol. 13, 605-611, 2003 |
| (4) |
Kitazawa K, Nadanaka S, Kadomatsu K, Kitagawa H: Chondroitin 6-sulfate represses keratinocyte proliferation in mouse skin, which is associated with psoriasis. Commun. Biol. 4, 114, 2021 |
| (5) |
Koike T, Mikami T, Tamura JI, Kitagawa H: Altered sulfation status of FAM20C-dependent chondroitin sulfate is associated with osteosclerotic bone dysplasia. Nat. Commun. 13, 7952, 2022 |
Jun. 15, 2023
|
|---|







