|
Glycosyl Photoaffinity Probes: Synthesis and
Application
|
|
|
 |
One of the major events occurring at biological interfaces
is the specific recognition of bioactive ligands by their receptor proteins.
The method of photoaffinity labeling enables the direct probing of target
protein through a covalent bond introduced between a ligand and its specific
receptor (1).
The first step in photoaffinity labeling is the synthesis of photoreactive
probes by attaching a photoreactive group on a ligand molecule such as
a carbohydrate. Among photoreactive groups currently used, diazirine meets
most of the criteria for an ideal photoreactive group. In addition to
its super reactivity and the stability of cross-link, diazirines can be
rapidly photolyzed at wavelengths beyond the UV absorbance region of proteins.
Several biotinylated diazirines were developed as a non-radioisotopic
solution to the laborious photoaffinity labeling routine (Figure).
Recently, a biotinylated diazirine with an aminooxy group (R = CH2ONH2)
was developed for the protection-free preparation of carbohydrate photoprobes
from the micromole quantities of various oligosaccharides. By a single-step
reaction, a biotinylated carbene-generating unit can be introduced to
the reducing end of unprotected carbohydrates. By using the commercially
available biotinyl diazirine (Seikagaku Corporation), a sequence of photoaffinity
labeling experiments, from the probe synthesis to the detection of labeled
protein, could be readily accomplished within one week (2).
Photoaffinity labeling can be applied at two levels (Figure).
At the protein level, the cross-link is useful for the identification
of target proteins by a SDS-PAGE analysis (bottom left). If the binding
site analysis of the target protein is important, the photoaffinity labeling
will give sequence information on binding site peptides (bottom right).
Recent developments in mass spectroscopy provide an efficient way for
the analysis of peptide sequences.
Major approaches currently used in the investigation of protein functional
sites have their own advantages and limitations. The genetic approach,
for example, provides a series of mutants for the structural analysis
of functional sites. However, it is generally impossible to exclude potential
conformational changes that may result in alterations of ligand binding
processes. Thus, photoaffinity labeling is a reliable chemical method
which should be considered as being complementary to, rather than in competition
with other approaches. A biotinylated photoaffinity probe was applied
in the analysis of acceptor binding-site within 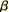 1,4-galactosyltransferase.
The photochemically introduced biotin tag harnesses the power of avidin-biotin
technology for one-step purification of photolabeled peptides from the
protein digest. The approach yielded, for the first time, information
on the acceptor site peptides in this enzyme. A molecular docking study
based on this fragment strongly suggests an active site carboxylate which
could be involved in the galactosyl transfer step. The result also demonstrates
that the combination of photoaffinity labeling, crystallography and 3D
graphics can be a powerful solution for detailed structural analysis of
ligand-protein complexes (3). 1,4-galactosyltransferase.
The photochemically introduced biotin tag harnesses the power of avidin-biotin
technology for one-step purification of photolabeled peptides from the
protein digest. The approach yielded, for the first time, information
on the acceptor site peptides in this enzyme. A molecular docking study
based on this fragment strongly suggests an active site carboxylate which
could be involved in the galactosyl transfer step. The result also demonstrates
that the combination of photoaffinity labeling, crystallography and 3D
graphics can be a powerful solution for detailed structural analysis of
ligand-protein complexes (3).
|
|
|
|
Yasumaru HATANAKA
(Faculty of Pharmaceutical Sciences,
Toyama Medical and Pharmaceutical University) |
|
|
 |
|
|
| References |
(1) |
Hatanaka Y.; Sadakane Y., Photoaffinity Labeling in Drug Discovery
and Developments: Chemical Gateway for Entering Proteomic Frontier,
Curr. Top. Med. Chem., 2, 271-288, 2002. |
|
(2) |
Hatanaka, Y.; Kempin, U.; Park, J. -J., One-step Synthesis of
Biotinyl Photoprobes from Unprotected Carbohydrates. J. Org.
Chem., 65, 5639-5643, 2000. |
|
(3) |
Hatanaka, Y.; Ishiguro, M.; Hashimoto, M.; Gastinel, L. N.; Nakagomi,
K., A Model of Photoprobe Docking with  1,4-Galactosyltransferase
Identify a Possible Carboxylate Involved in Glycosylation Steps.
Bioorg. Med. Chem. Lett., 11, 411-413,
2001. 1,4-Galactosyltransferase
Identify a Possible Carboxylate Involved in Glycosylation Steps.
Bioorg. Med. Chem. Lett., 11, 411-413,
2001. |
|
|
|
|
|
| Apr. 1, 2003 |
|
|
|
|
|
|
|



