|
Metabolic Cytometry: Monitoring Oligosaccharide Biosynthesis in Single Cells
|
|
|
 |
Metabolism is the total set of chemical reactions that occur within a cell. Current methods for monitoring cellular metabolism require that a large number of cells be analyzed using methods such as spectroscopy, chromatography and electrophoresis. Detailed information about metabolic pathways can be obtained with these methods. However, since these are bulk assays that are averaged over many cells they cannot provide information on cell to cell variation. In contrast, cytometric assays such as flow cytometry and fluorescence microscopy can be used to analyze chemical and physical properties of individual cells. In flow cytometry a cell suspension flows past a focused laser beam; light scatter and fluorescence are used to characterize the size and chemical composition of the cell. Cell-surface markers can be detected using of fluorescently labeled antibodies, cell cycle phase can be estimated using of DNA intercalating dyes, and a few intra-cellular enzymes can be monitored using of fluorogenic substrates. However, since there are few distinct fluorogenic substrates available and limited spectral channels it is difficult to monitor multiple enzyme activities with in a single cell.
The new technique of metabolic cytometry can be used to monitor metabolic oligosaccharide conversions in individual cells (1,2). This is an ultra-sensitive method that employs capillary electrophoresis as the final analytical step. In metabolic cytometry, cells are grown in the presence of a fluorescently tagged oligosaccharide substrate. Substrate is taken up by the cells and intracellularly converted to a series of reaction products. Conversions are monitored by capillary electrophoresis and laser-induced fluorescence detection after introducing a single cell into the separation capillary with a micromanipulator (1). As few as 100 molecules of reaction product can be detected.
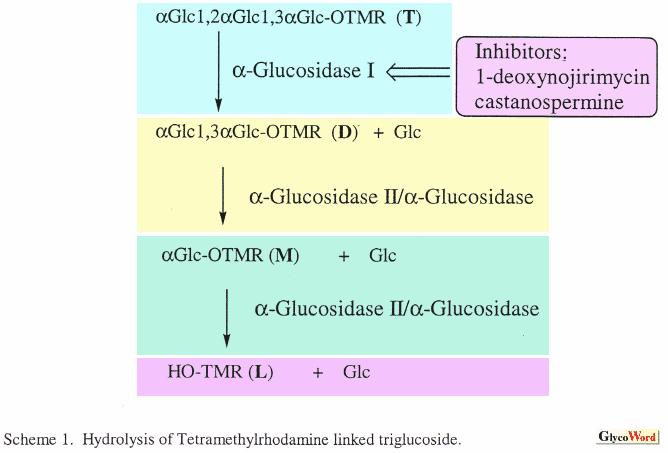
One application of metabolic cytometry utilizes alphaGlc1,2alpha Glc1,3alphaGlc-TMR substrate, a triglucoside covalently linked to tetramethylrhodamine via an ethylendiamine linker (3) to monitor hydrolytic pathways in yeast or mammalian cells. This substrate is taken up by the cells and converted to disaccharide alphaGlc1,3alphaGlc-TMR (D) by the endoplasmic reticulum localized alpha-glucosidase I (Scheme 1). The disaccharide can be further hydrolyzed to monsaccharide alphaGlc-TMR (M) and the TMR-linking arm (L). Electropherograms of single yeast spheroplasts (Fig. 1) show approximately 500-1000 molecules of TMR-labeled trisaccharide and the free linker arm with less than 1000 molecules of the intermediate disaccharide and monosaccharide (Fig. 2). This conversion is inhibited by pre-incubation of the yeast cells with castanospermine, a competitive inhibitor of alpha-glucosidase I (2).
|
|
|
 |
 |
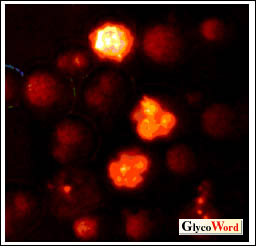 |
Fig. 1. Confocal microscopy of yeast cells incubated
with 50  M Triglucoside-TMR for 24 h. M Triglucoside-TMR for 24 h. |
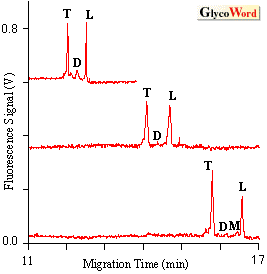 |
Fig. 2. Electropherograms of the contents of
three individual yeast spheroplasts.
|
|
|
|
|
Single cell biosynthesis and hydrolysis employing betaGal1,4betaGlcNAc-TMR (LacNAc-TMR) has also been investigated in HT-29 cells (2). This disaccharide is taken up by cells and converted to a series of products. These include betaGlcNAc-TMR by enzymatic hydrolysis of the substrate and both the monofucosylated trisaccharide Lex-TMR and the difucosylated tetrasaccharide Ley-TMR catalyzed by fucosyltransferases in the Golgi apparatus. On an individual cellular basis, either Lex or Ley synthesis, but rarely both, were observed suggesting controlled substrate flux through the Golgi appatatus.
Metabolic cytometry is not restricted to oligosaccharide metabolism; it can be used whenever a cell takes up a fluorescent enzyme substrate. This technology should find broad applications including those in which the limited cell numbero of cells(embryogenesis, biopsy) has precluded analysis.
|
|
|
|
|
|
Monica M. Palcic@(Department of Chemistry, University of Alberta) |
|
|
|
|
|
| References |
(1) |
Le XC, Tan W, Scaman CH, Szpacenko A, Arriaga E, Zhang Y, Dovichi NJ, Hindsgaul O, Palcic MM@: Single cell studies of enzymatic hydrolysis of a tetramethylrhodamine labeled triglucoside in yeast. Glycobiology 9, 219-225, 1999
|
|
(2) |
Krylov SN, Zhang Z, Chan NWC, Arriaga E, Palcic MM, Dovichi NJ: Correlating cell cycle with metabolism in single cells : the combination of image and metabolic cytometry. Cytometry volume 37, page14-20, in 1999
|
|
(3) |
Scaman CH, Hindsgaul O, Palcic MM, Srivastava OP: Synthesis of alpha-D-Glcp-(1¨2)- alpha- D-Glcp-(1¨3)- alpha-D-Glcp-O-(CH2)8COOCH3 for use in the assay of alpha-glucosidase I activity. Carbohydr. Res. 296, 203-213, 1996
|
|
|
|
|
|
| Revised; Sep. 15, 1999 |
|
|
|
|
|
|



