|
Efficient Methods for Synthesizing Oligosaccharide Units and Derivatives Utilizing Lysozyme and Cellulase
|
|
|
 |
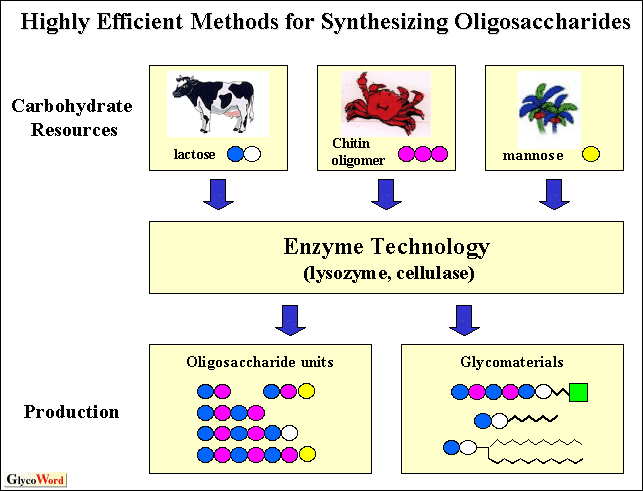
We descibe herewith an efficient enzymatic method for synthesizing useful oligosaccharides and derivatives utilizing lysozyme-mediated and cellulase-mediated transglycosylation.
I. Lysozyme-mediated transglycosylation
p-Nitorophenyl penta-N-acetyl- -chitopentaoside (pNP- -chitopentaoside (pNP- -(GlcNAc)5), which is available as a substrate of lysozyme assay, was efficietly synthesized through hen egg-white lysozyme-mediated transglycosylatrion from penta-N-acetylchitopentaose (GlcNAc)5 to the OH-4 position of p-nitrophenyl -(GlcNAc)5), which is available as a substrate of lysozyme assay, was efficietly synthesized through hen egg-white lysozyme-mediated transglycosylatrion from penta-N-acetylchitopentaose (GlcNAc)5 to the OH-4 position of p-nitrophenyl  -N-acetylglucosaminide (pNP- -N-acetylglucosaminide (pNP- -GlcNAc) in an aqueous solution containing dimethylsulfoxide (Me2SO) in a high concentration.1) It was obtained in a high yield of 46% based on the (GlcNAc)5 donor added. The desired compound insolubilized from the reaction mixture was directly obtained in a high state of purity by washing with aqueous methanol only without using any column chromatographic separation. As a result, the addition of Me2SO to the reaction system not only ensured the solubility of pNP- -GlcNAc) in an aqueous solution containing dimethylsulfoxide (Me2SO) in a high concentration.1) It was obtained in a high yield of 46% based on the (GlcNAc)5 donor added. The desired compound insolubilized from the reaction mixture was directly obtained in a high state of purity by washing with aqueous methanol only without using any column chromatographic separation. As a result, the addition of Me2SO to the reaction system not only ensured the solubility of pNP- -GlcNAc, but also resulted in a high yield of pNP- -GlcNAc, but also resulted in a high yield of pNP- -(GlcNAc)5. -(GlcNAc)5.
Hen egg-white lysozyme also had GlcNAc transferase activity and was utilized to synthesize the 4-O- -N-acetylglucosaminyl-mannnose (GlcNAc -N-acetylglucosaminyl-mannnose (GlcNAc 1-4Man) sequence present in N-linked glycan. The enzyme produced GlcNAc 1-4Man) sequence present in N-linked glycan. The enzyme produced GlcNAc 1-4Man through a regioselective transglycosylation from N,N’-diacetylchitobiose (GlcNAc)2 and Man in a yield of 20.9 % based on the donor added.2) It was capable of transferring an N-acetylglucosaminyl group from the donor exclusively to the OH-4 of the Man moiety. This was also the case for the synthesis of GlcNAc 1-4Man through a regioselective transglycosylation from N,N’-diacetylchitobiose (GlcNAc)2 and Man in a yield of 20.9 % based on the donor added.2) It was capable of transferring an N-acetylglucosaminyl group from the donor exclusively to the OH-4 of the Man moiety. This was also the case for the synthesis of GlcNAc 1-4Man 1-4Man -OC6H4NO2-p with p-nitorophenyl -OC6H4NO2-p with p-nitorophenyl  -D-mannopyranoside as an acceptor. It was obtained in a yield of 10.5 % based on the donor added. -D-mannopyranoside as an acceptor. It was obtained in a yield of 10.5 % based on the donor added.
II. Cellulase-mediated transglycosylation
Our interest was directed to an enzymatic approach involving a lactosyl unit  -glycosidically linked to aliphatic groups as mimic units of glycosphingolipids. Aliphatic -glycosidically linked to aliphatic groups as mimic units of glycosphingolipids. Aliphatic  -lactosides were conveniently synthesized by -lactosides were conveniently synthesized by  -lactosyl transfer reaction from Lac -lactosyl transfer reaction from Lac -pNP donor to various 1-alkanols (n = 2~12) accetors, utilizing cellulase from Trichoderma reesei. With ethanol acceptor, the enzyme induced ethyl -pNP donor to various 1-alkanols (n = 2~12) accetors, utilizing cellulase from Trichoderma reesei. With ethanol acceptor, the enzyme induced ethyl  -lactoside in 18% yield based on the donor added in an aqueous system. However, the yield decreased as the alkyl chain of alkanol acceptors increased from C2 to C8. With octanol acceptor, octyl -lactoside in 18% yield based on the donor added in an aqueous system. However, the yield decreased as the alkyl chain of alkanol acceptors increased from C2 to C8. With octanol acceptor, octyl  -lactoside was observed in a low yield (<1%). This problem was partially solved by using sodium cholate as the detergent. When 1-octanol and 1-dodecanol acceptors were incubated in the presence of sodium cholate, the transfer products octyl -lactoside was observed in a low yield (<1%). This problem was partially solved by using sodium cholate as the detergent. When 1-octanol and 1-dodecanol acceptors were incubated in the presence of sodium cholate, the transfer products octyl  -lactoside and dodecyl -lactoside and dodecyl  -lactoside were obtained in 13 and 5% yields respectively based on the donor added. The addition of sodium cholate to the reaction system not only guaranteed solubility of the substrate, but also resulted in a remarkable increase in the yield. These enzyme reactions were efficient enough to allow one-pot preparation of the desired lactoside derivatives.3) -lactoside were obtained in 13 and 5% yields respectively based on the donor added. The addition of sodium cholate to the reaction system not only guaranteed solubility of the substrate, but also resulted in a remarkable increase in the yield. These enzyme reactions were efficient enough to allow one-pot preparation of the desired lactoside derivatives.3)
The enzyme also had LacNAc transferring activity and was utilized to synthesize part of the antennary structure of an N-linked sugar chain. Preparative scale syntheses were done utilizing Man and Glc as acceptors with a LacNAc -pNP donor. As a result, the enzyme transferred an N-acetyllactosamine unit from the donor exclusively to the OH-4 of Man and Glc moieties. The compounds Gal -pNP donor. As a result, the enzyme transferred an N-acetyllactosamine unit from the donor exclusively to the OH-4 of Man and Glc moieties. The compounds Gal 1-4GlcNAc 1-4GlcNAc 1-4Man and Gal 1-4Man and Gal 1-4GlcNAc 1-4GlcNAc 1-4Glc were obtained in 13 and 9% yields respectively based on the donor added. These products were conveniently isolated by one-step chromatographic separation on a charcoal-Celite column.3) 1-4Glc were obtained in 13 and 9% yields respectively based on the donor added. These products were conveniently isolated by one-step chromatographic separation on a charcoal-Celite column.3) |
|
|
 |
|
|
Taichi Usui
(Faculty of Agriculture, Shizuoka University) |
|
|
|
|
|
| References |
(1) |
T, Usui : Regioselective synthesis of useful oligosaccharide by glycosidase, Trends in Glycosci. Glycotechnol. 4, 116-122, 1992 |
|
(2) |
T, Murata T, Usui : Enzymatic synthesis of important oligosaccharide units involved in N- and O-linked glycans, Trends in Glycosci. Glycotechnol. 12, 161-174, 2000 |
|
(3) |
K, Totani N, Yasutake H, Ohi T, Murata T, Usui : Enzymatic synthesis of aliphatic  -lactosides as mimic units of glycosphingolipids by use of Trichoderma reesei cellulase, Arch. Biochem. Biophys. 385, 70-77, 2001 -lactosides as mimic units of glycosphingolipids by use of Trichoderma reesei cellulase, Arch. Biochem. Biophys. 385, 70-77, 2001 |
|
|
|
|
|
| Mar.15, 2002 |
|
|
|
|
|
|
|



