 |
O-glycan is very difficult to synthesize chemically because it contains long and branched carbohydrate chains. However our group has developed an enzymatic methods for the synthesis of O-glycans easily and efficiently. There are two practical methods for synthesizing a glycopeptide using endo-type glycosidase. One is to combine the chemical synthesis of glycosyl peptide with the enzymatic method of transglycosylation of endo-type glycosidase (chemoenzymatic synthesis),and the other is enzymatic synthesis using transglycosylation activity of endo-type glycosidase. Here we describe that a practical method for synthesizing a glycopeptide containing O-linked oligosaccharides using transglycosylation activity of only endo- -N-acetylgalactosaminidase (endo- -N-acetylgalactosaminidase (endo- -GalNAc-ase). Endo- -GalNAc-ase). Endo- -GalNAc-ase hydrolyzes the O-glycosidic linkage between N-acetyl-D-galactosamine and serine(threonine) in O-glycan, liberating oligosaccharides. The enzyme was found in the culture fluid of Clostridium perfringens, Diplococcus pneumoniae, Alcaligenes sp., Bacillus sp. and Streptomyces sp., and the substrate specificities and the aglycone specificities of these enzymes are slightly different. -GalNAc-ase hydrolyzes the O-glycosidic linkage between N-acetyl-D-galactosamine and serine(threonine) in O-glycan, liberating oligosaccharides. The enzyme was found in the culture fluid of Clostridium perfringens, Diplococcus pneumoniae, Alcaligenes sp., Bacillus sp. and Streptomyces sp., and the substrate specificities and the aglycone specificities of these enzymes are slightly different.
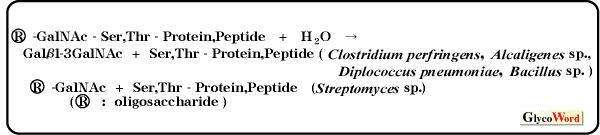
As these endo- -GalNAc-ases liberate O-linked oligosaccharide from cell surface glycoproteins without causing damage to cells, they are useful for the investigation of the structure and function of O-linked oligosaccharides on the cell surface. Endo- -GalNAc-ases liberate O-linked oligosaccharide from cell surface glycoproteins without causing damage to cells, they are useful for the investigation of the structure and function of O-linked oligosaccharides on the cell surface. Endo- -GalNAc-ase from Diplococcus pneumoniae (endo- -GalNAc-ase from Diplococcus pneumoniae (endo- -GalNAc-ase-D) and from Alcaligenes sp. (endo- -GalNAc-ase-D) and from Alcaligenes sp. (endo- -GalNAc-ase-A) have frequently been used as an important reagent in studies on the structure and physiological role of O-linked oligosaccharides. The endo-GalNAc-ases from Diplococcus pneumoniae and Bacillus sp. show transglycosylation activity and can transfer the oligosaccharides (Gal -GalNAc-ase-A) have frequently been used as an important reagent in studies on the structure and physiological role of O-linked oligosaccharides. The endo-GalNAc-ases from Diplococcus pneumoniae and Bacillus sp. show transglycosylation activity and can transfer the oligosaccharides (Gal 1-3GalNAc) from glycoside donor to various acceptors, such as monosaccharides, disaccharides, sugar alcohols, Tris, glycerol, p-nitrophenol, threonine and serine, during hydrolysis of the glycoside donor. 1-3GalNAc) from glycoside donor to various acceptors, such as monosaccharides, disaccharides, sugar alcohols, Tris, glycerol, p-nitrophenol, threonine and serine, during hydrolysis of the glycoside donor.

The donor specificities and the acceptor specificities on the transglycosylation activity of these enzymes are slightly different. Then, using the transglycosylation activity of endo- -GalNAc-ase from Streptomyces sp., glycopeptide is enzymatically synthesized (Fig. 1). The method in the synthesis is the addition of oligosaccharide from a glycosyl donor ( glycoprotein, glycopeptide, and/or oligosaccharide derivative ) to peptide using the transglycosylation activity of endo- -GalNAc-ase from Streptomyces sp., glycopeptide is enzymatically synthesized (Fig. 1). The method in the synthesis is the addition of oligosaccharide from a glycosyl donor ( glycoprotein, glycopeptide, and/or oligosaccharide derivative ) to peptide using the transglycosylation activity of endo- -GalNAc-ase. The reaction mixture was applied to a preparative reverse phase HPLC column to give oligosaccharide peptide. In order to reach a more decisive conclusion about the structure of the glycopeptide, 1H-NMR spectral data and MS spectral data were collected and analyzed. The total yield of the glycopeptide via this process is better than that of the chemoenzymatic method (Fig. 2). Although free peptides are hydrolyzed within 1 hr, peptides with attached oligosaccharide show remarkable resistance to hydrolysis. As endo- -GalNAc-ase. The reaction mixture was applied to a preparative reverse phase HPLC column to give oligosaccharide peptide. In order to reach a more decisive conclusion about the structure of the glycopeptide, 1H-NMR spectral data and MS spectral data were collected and analyzed. The total yield of the glycopeptide via this process is better than that of the chemoenzymatic method (Fig. 2). Although free peptides are hydrolyzed within 1 hr, peptides with attached oligosaccharide show remarkable resistance to hydrolysis. As endo- -GalNAc-ase from Streptomyces sp. has wide substrate specificity, the present method can be applied to the synthesis of a wide variety of O-glycosidic linkage glycopeptides. -GalNAc-ase from Streptomyces sp. has wide substrate specificity, the present method can be applied to the synthesis of a wide variety of O-glycosidic linkage glycopeptides.
|
|
|
| References |
(1) |
Bardales R and Bhavanandan VP : Transglycosylation and transfer reaction activities of endo- -N-acetyl-D-galactosaminidase from Diplococcus -N-acetyl-D-galactosaminidase from Diplococcus
(Streptococcus ) pneumoniae. J. Biol. Chem. 264, 19893-19897, 1989 |
|
(2) |
Ashida H, Yamamoto K, Murata T, Usui T and Kumagai H : Characterization of endo- -N-acetylgalactosaminidase from Bacillus sp. and syntheses of neo- -N-acetylgalactosaminidase from Bacillus sp. and syntheses of neo-
oligosaccharides using its transglycosylation activity. Arch. Biochem. Biophys. 373, 394-400, 2000 |
|
(3) |
Ishii-Karakasa I, Iwase H, Hotta K, Tanaka Y and Omura S : Partial purification and characterization of an endo- -N-acetylgalactosaminidase from the culture medium of Streptomyces sp. OH-11242. Biochem. J. 288, 475-482, 1992 -N-acetylgalactosaminidase from the culture medium of Streptomyces sp. OH-11242. Biochem. J. 288, 475-482, 1992 |
|
(4) |
Ajisaka K, Miyasato M and Ishii-Karakasa I : Efficient synthesis of O-linked
glycopeptide by a transglycosylation using endo- -N-acetylgalactosaminidase from Streptomyces sp. Biosci. Biotechnol. Biochem. 65, 1240-1243, 2001 -N-acetylgalactosaminidase from Streptomyces sp. Biosci. Biotechnol. Biochem. 65, 1240-1243, 2001 |
|
|



