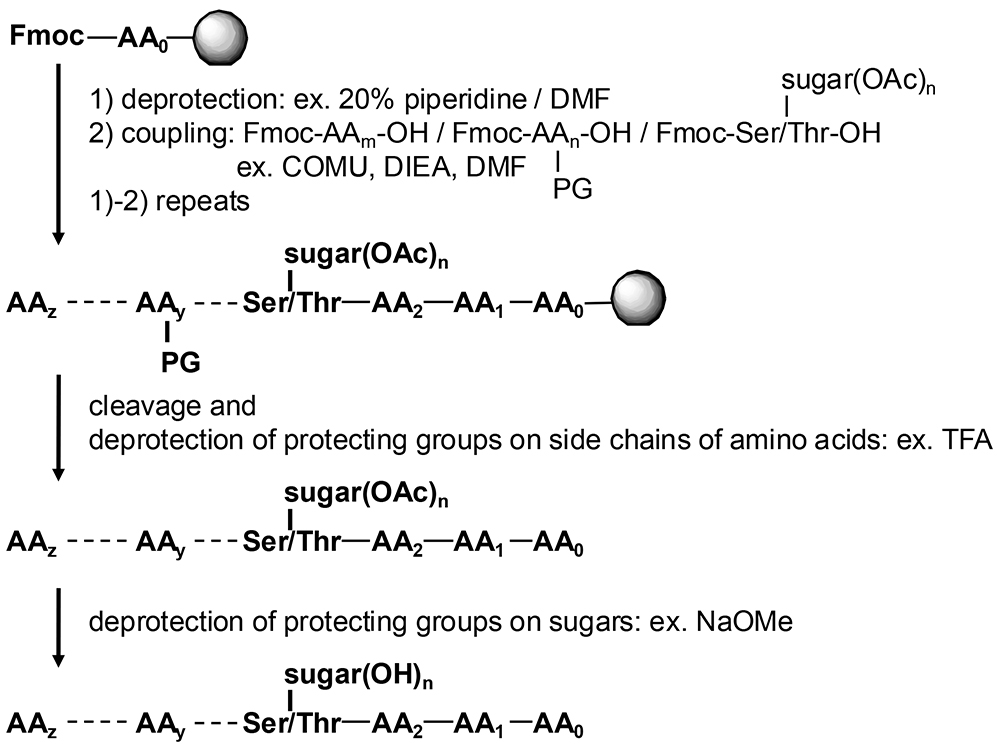
Microwave Application for Chemical Synthesis of Glycopeptide | |||||||||||||||
 |
Glycopeptides are molecules having glycans and peptide chains, which play an important role in biological functions and pharmacological actions. Since most of animal functional proteins are glycosylated, these substructures have attracted much attention in the fields of vaccines, anticancer drugs, and diagnostic agents.
In nature, glycans on glycoproteins are not homogeneous, and it is difficult to separate and purify them.
Matsushita et al. achieved the synthesis of a twenty amino acid residual glycopeptide, a MUC1 repeating unit with five of trisaccharides, by heating the reaction temperatures to 50℃ by microwave irradiation. They compared three reaction conditions, namely at room temperature (25℃), at 50℃ by conventional heating and at 50℃ by microwave, then microwave heating at 50℃ gave the best results, total yield 44% and total reaction time 7 hours. The synthesis under totally same conditions with the case of using microwave but heated by conventional heating at 50℃, the total reaction time was indeed 7 hours but the total yield was only 15%. They discussed that these results indicated some kind of microwave unique effect (1,2). Despite their discussion, the data obtained might not be to reflect the microwave effect because their experiments were not performed on the same scale and with the same equipment. Ohki et al. developed a microwave synthesizer with a cooling unit that could investigate the microwave effect (3). Yokoe et al. demonstrated the synthesis of a five amino acid residual peptide. The overall yield was increased under microwave irradiation and they also reported that only 1.3 equivalents of glyco-amino acid synthons, which are usually used in large excess, were required (3). Thus, the advantages of microwave irradiation are not only to obtain high yields but also to reduce of the essential amount of reagents. Shimizu et al. have successfully synthesized complex glycopeptides containing galactosamine monosaccharide, Tn antigen, Sia-Gal-GalNAc trisaccharide, ST antigen, and Sia-Gal-(Sia)GalNAc tetrasaccharide, diSiaT antigen and they are now developing drug discovery research (unpublished). Hojo et al. introduced benzyl-protected diSiaT tetrasaccharide amino acid at room temperature, and performed further extension for a few residues by microwave heating to 90℃ (4). Although sialic acid-containing glyco-amino acid synthons are considered to be extremely unstable, they are relatively heat stable when incorporated into peptide chains. Although the mechanism and details of how microwave can provide non-thermal effects are still under study, Nagashima et al. investigated the microwave effect by changing the frequency of irradiated microwaves for enzymatic hydrolysis (5). Microwave heating is mainly caused by the dielectric effect of microwaves on water molecules which are dipole molecules, but they discussed that this enzymatic reaction was accelerated by the conductive effect, i.e. microwaves selectively affected ions. They discussed that one of the microwave effects was the molecular/ion selective affection. The author also considers that the contribution of microwaves to hydrogen bonding networks for substrates and/or enzymes may also produce the microwave effect. Some of the academic reports for glycopeptide syntheses have tended to be qualitative or at the sub-mg level due to the difficulty in obtaining reagents, and there have been very few reports discussing details of reaction conditions and stability studies. On the other hand, the need for glycopeptide synthesis is expected to increase in the research fields of protein science and glycoscience, as well as in drug discovery. It has become clear that the use of microwaves, even at low temperatures, offers advantages, and the synthesis of O-glycan glycopeptides such as the diSiaT series, which are complex and difficult to synthesize, is now becoming more feasible. Microwaves are expected to be one of the ways to break through this situation.
Hiroki Shimizu
| ||||||||||||||
| References | |
|---|---|
| (1) | Matsushita T, Hinou H, Kurogochi M, Shimizu H, Nishimura SI: Rapid Microwave-Assisted Solid-Phase Glycopeptide Synthesis. Org. Lett. 7, 877-880, 2005 |
| (2) | Matsushita T, Hinou H, Fumoto M, Kurogochi M, Fujitani N, Shimizu H, Nishimura SI: Construction of Highly Glycosylated Mucin-Type Glycopeptides Based on Microwave-Assisted Solid-Phase Syntheses and Enzymatic Modifications. J. Org. Chem. 71, 3051-3063, 2006 |
| (3) | Yokoe T, Ohki Y, Nagashima I, Takahashi N, Shimizu H: Study of Glycopeptide Synthesis under Microwave Irradiation at Low Temperatures towards Automated Synthesiser. JEMEA J. 3, 32-39, 2019 |
| (4) | Nagashima K, Ito S, Takei T, Takao T, Kiyozumi D, Hojo H: Synthesis and Structural Analysis of NICOL with O-Linked Glycosylation. The 61st Japanese Peptide Society, P-023, 2024 |
| (5) | Nagashima I, Sugiyama Ji, Shimizu H: Study of 400 MHz microwave conduction loss effect for a hydrolysis reaction by thermostable β-Glucosidase HT1. Biosci. Biotechnol. Biochem. 87, 158-162, 2023 |
Mar. 3, 2025