 |
Sialic acids refer to a family of nine carbon monosaccharides based on α-keto carboxylic acid (Fig. 1). Among these, the sialic acid 1 with an amino group is known as neuraminic acid. Naturally occurring forms include N-acetylneuraminic acid (NeuAc 2) and N-glycolylneuraminic acid (NeuGc 3), both of which play crucial biological roles. Therefore, the synthesis of glycans containing sialic acid is a significant challenge in glycan synthesis chemistry. Particularly, α(2,8)-disialic acid 4, which is α-linked via a hydroxyl group at the C8 position, and its oligomers or polymers are recognized as some of the most challenging glycans to synthesize due to the complexity of the chemical synthesis involved. To efficiently synthesize these glycans, the following challenges must be addressed:
1. Establishing highly efficient α-sialylation reactions with high stereoselectivity.
2. Development of glycosyl acceptors suitable for glycosylation at the low-reactivity 8-position hydroxyl group.
3. Synthesizing derivatives with various N-acyl groups involving acetyl or glycolyl groups.
To overcome these challenges, sialyl donor 5 with a cyclic carbonyl protecting group at the C4 hydroxyl group and the C5 amino group was developed. These glycosyl donors are categorized into three generations based on their development.
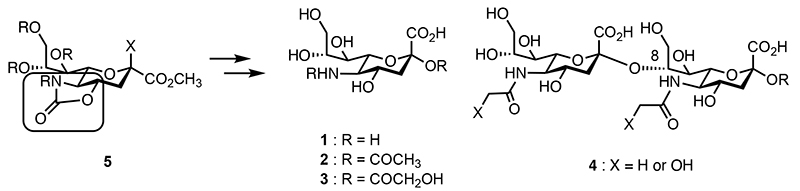
Fig. 1. Structures of sialosides 1-4 and the sialyl donor 5 with a cyclic carbonyl protecting group
|
First Generation: 4N,5O-Carbonyl Type (1,2)
Glycosyl donor 6 is the foundational form in this series (Fig. 2). Glycosyl donor 6 selectively provides α-sialoside 7 with high stereoselectivity even in non-nitrile solvents such as methylene chloride. However, since α-sialoside 7 lacks a nitrogen substituent, it is necessary to introduce a nitrogen substituent during the deprotection process to obtain the unprotected sialoside 8 after glycan assembly. This design enables the synthesis of diverse nitrogen-substituted derivatives, including non-natural types, from a single intermediate. Using this glycosyl donor, the first chemical syntheses of α(2,8) tetrasialic acid and the glycan part of ganglioside GP1c containing five sialic acid residues have been achieved. However, introducing nitrogen substituents after glycan assembly increases the number of steps, reducing the overall efficiency. Additionally, when synthesizing oligosaccharides containing sialic acids with different nitrogen substituents, separate transformations for each sialic acid are required.
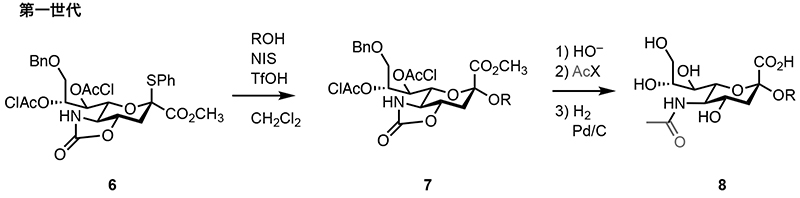
Fig. 2. Synthesis of sialoside 8 using first-generation sialyl donor 6.
|
Second Generation: N-Acetyl-4N,5O-Carbonyl Type (3)
Glycosyl donor 9 contains cyclic carbonyl protecting groups, including an N-acetyl group. The resulting glycoside 10 can be selectively deprotected under basic conditions to directly yield N-acetyl sialic acid 12. However, when using a glycolyl-containing donor 11, de-glycolylation progresses under basic conditions, leading to the formation of the amine 13. Additionally, the N-acetylated donor 9 exhibits reduced α-selectivity and requires the addition of nitrile solvents for glycosylation reactions involving secondary alcohols.
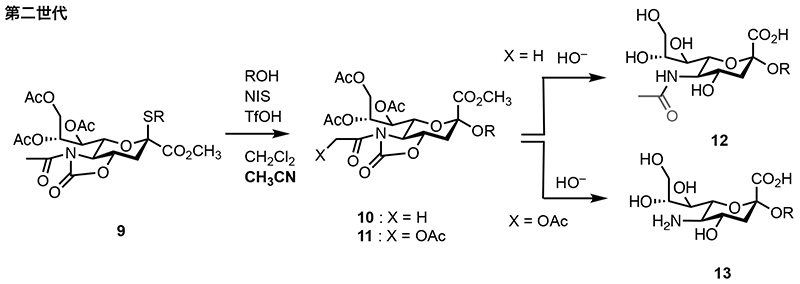
Fig. 3. Synthesis of sialoside 12 using second-generation sialyl donor 9.
|
Third Generation: N-Acyl-O,9O:4N,5O-Dicarbonyl Type (4,5)
Glycosyl donor 14 features cyclic carbonyl protecting groups at the 7- and 9-position hydroxyl groups in addition to the 4N,5O positions. These side-chain carbonyl groups further enhance α-selectivity, enabling high α-selectivity for secondary alcohols even in dichloromethane solvents. They also contribute to increased reactivity of the 8-position hydroxyl group. This donor has enabled the synthesis of an octasialic acid, the smallest repeating unit of polysialic acids. Furthermore, selective ring-opening reactions of N-acyl cyclic carbamates in glycoside 15 using thiols have been developed, allowing the direct synthesis of not only N-acetyl sialic acid but also N-glycolyl sialic acid 16.
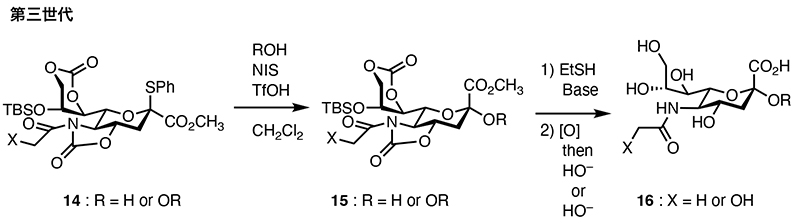
Fig. 4. Synthesis of sialoside 14 using second-generation sialyl donor 16.
|
In summary, the 4N,5O-carbonyl protecting group significantly enhances the α-selectivity of sialic acid as a glycosyl donor. The glycosyl donors have been utilized not only in the synthesis of α(2,8) oligo- and polysialic acids but also in a variety of glycan syntheses.
Hiroshi Tanaka
(Faculty of Pharmacy, Juntendo University)
| References |
| (1) |
Tanaka H, Nishiura Y, Takahashi T: Stereoselective synthesis of oligo-α-(2,8)-sialic acids. J. Am. Chem. Soc. 128, 7124–7125, 2006 |
| (2) |
Tanaka H, Nishiura Y, Takahashi T: An efficient convergent synthesis of GP1c ganglioside epitope. J. Am. Chem. Soc. 130, 17244–17245, 2008 |
| (3) |
Crich D, Li W: O-Sialylation with N-acetyl-5-N,4-O-carbonyl-protected thiosialoside donors in dichloromethane: facile and selective cleavage of the oxazolidinone ring. J. Org. Chem. 72, 2387–2391, 2007 |
| (4) |
Koinuma R, Tohda K, Aoyagi T, Tanaka H: Chemical synthesis of α(2,8) octasialosides, the minimum structure of polysialic acids, Chem. Commun. 56, 12981–12984, 2020 |
| (5) |
Takeuchi Y, Tohda K, Tanaka H: Syntheses of α(2,8) Sialosides containing NeuAc and NeuGc by using double carbonyl-protected N-acyl sialyl donors. Chem. Eur. J. 30, e202400883, 2024 |
Mar. 3, 2025
|
|---|










