|
Synthesis of Biologically Active Carba-Sugar Derivatives
|
 |
|
 |
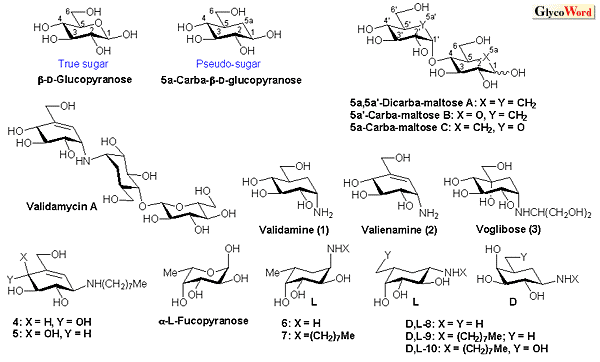
Carba-D-glucopyranose is a carbocyclic (cyclohexane) analogue of D-glucopyranose in which the ring oxygen atom is replaced by a methylene group, and it belongs to what are known as pseudo-sugars. Azasugars, thiosugars, etc. are other pseudo-sugars. Their structures resemble very much those of the corresponding true sugars and some possess interesting biological activity.
In 1966, McCasland first synthesized carba-sugar and noted : “It is hoped that pseudo-sugars may be found acceptable in place of true sugars to some but not all enzymes or biological systems, and thus might serve to inhibit growth of malignant or pathogenic cells.” Soon after his prediction, in 1970, agricultural antibiotic validamycin A composed of carba-sugars was found in Japan, and subsequently, in 1977, production of antidiabetic agent acarbose was disclosed in Germany. In the course of studies on the structure–activity relationship of these natural substances, carba-sugar amines validamine 1 and valienamine 2 were isolated and shown to exhibit strong inhibitory activity against some  -glucosidases. Extensive chemical modification of these amines then led to the discovery of voglibose 3 comparable to acarbose. Their glycosidase inhibitory activity may be due to the striking resemblance in their structures to those of the corresponding substrates postulated to be adopted in the ground and transition-state during enzymatic hydrolysis. -glucosidases. Extensive chemical modification of these amines then led to the discovery of voglibose 3 comparable to acarbose. Their glycosidase inhibitory activity may be due to the striking resemblance in their structures to those of the corresponding substrates postulated to be adopted in the ground and transition-state during enzymatic hydrolysis.
Although synthetic chemists first considered the natural compounds to be the targets of total synthesis, having achieved the goal of these projects, we have started to consider a promising future in which carba-sugars play important roles in glycobiology as faithful substitutes and/or skillful mimickings of true sugars.
There are three types of carba-oligosaccharides (Fig.1): taking maltose as an example, type A is composed of two carba-glucoses, types B and C consist of one true and one carba glucose. Types A and B, connected with ether-linkages, have the characteristic of being inactive to any glucosidase. Moreover, N-, S-linkages, etc. are possible for these types. In fact, N-linked carba-oligosaccharides of these types have been found in nature. |
|
|
 |
| Fig.1 |
|
|
Mimicking some naturally occurring disaccharides, e.g. maltose, lactose, trehalose, lactosamine, several N- and O-linked carba-disaccharides have been synthesized and subjected to limited biological assays. Some have been demonstrated to be substrate analogues or inhibitors of glycosidases and/or glycosyltransferases. Considering these results, it may be possible in the future to both improve the remarkable features of carba-oligosaccharides of biological interest and to design new compounds with various functions.
Recently, some glycosidase inhibitors composed of carba-glycosylamines have attracted much attention in the development of new medicines. N-Substituted 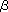 -valienamines 4 and 5 with -valienamines 4 and 5 with 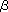 -gluco and -gluco and 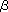 -galacto configurations were strong and specific inhibitors against the respective glycosylcerebrosidases. Alternatively, 5 has later been found to show a very strong activity towards human -galacto configurations were strong and specific inhibitors against the respective glycosylcerebrosidases. Alternatively, 5 has later been found to show a very strong activity towards human 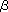 -galactosidase, leading possibly to the development of a new molecular therapy for human neurogenetic disease. N-Substituted validamines 6, 7 with the -galactosidase, leading possibly to the development of a new molecular therapy for human neurogenetic disease. N-Substituted validamines 6, 7 with the  -L-fucopyranose structure are very potent -L-fucopyranose structure are very potent  -fucosidase inhibitors, and its -fucosidase inhibitors, and its 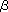 -anomers L-8, 9 interestingly still possess similar activity. However, it is worthy of note that its optical antipode D-9, the 6-deoxy derivative of the parent 5a-carba- -anomers L-8, 9 interestingly still possess similar activity. However, it is worthy of note that its optical antipode D-9, the 6-deoxy derivative of the parent 5a-carba-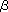 -galactopyranose derivative D-10, has been demonstrated to inhibit -galactopyranose derivative D-10, has been demonstrated to inhibit 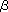 -galactosidase as well as -galactosidase as well as 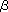 -glucosidase stronger than its parent. -glucosidase stronger than its parent.
In general, carba-oligosaccharides are stable in biological hydrolysis and are nontoxic in vivo. Therefore, they are important and reliable carbohydrate mimics that should be widely investigated in the near future. |
|
|
|
|
|
Seiichiro Ogawa (Department of Biosciences and Informatics, Faculty of Science and Technology, Keio University) |
|
|
|
|
|
| References |
(1) |
Ogawa S: "Design and synthesis of carba-sugars of biological interest" Trends Glycosci. Glycotechnol. 16, 33-53, 2004 |
|
(2) |
Ogawa S: “Synthesis of glycosidase inhibitors composed of carba-sugars.” Nippon Nogeikagaku Kaishi, 77, 974-981, 2003 |
|
(3) |
Ogawa S: “Synthetic Studies on Glycosidase Inhibitors Composed of 5a-Carba- Sugars,”in Carbohydrate Mimics (Y. Chapleur, ed.), Wiley-VCH, Weinheim, 87-106, 1998 |
|
(4) |
Ogawa S: “Synthetic Studies of Carba-Sugars of Biological Interest,” in Natural Products Chemistry, Vol.13 (A.-U. Rhaman, F. Z. Basha, ed.), Elsevier, Amsterdam, 187-255, 1993 |
|
|
|
|
|
| Apr. 23, 2004 |
|
|
|
|
|
|
|



