|
Systematic Synthesis of Gangliosides
|
 |
|
 |
Gangliosides are distinguished from other glycosphingolipids in that they contain sialic acid. Because the gangliosides are composed of three parts, sialic acid, oligosaccharide chain, and ceramide (Scheme), the crucial steps to synthesize the gangliosides are the introduction of sialic acid as well as ceramide to the oligosaccharide chain. It is also important to formulate a rational, synthetic strategy.
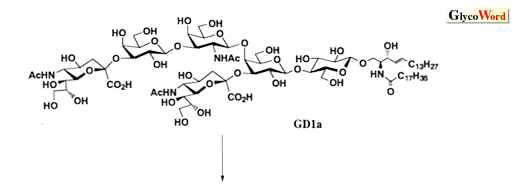
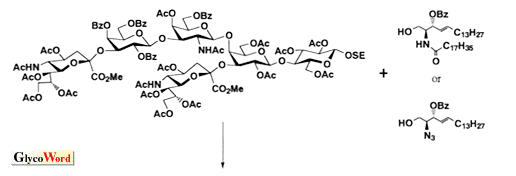
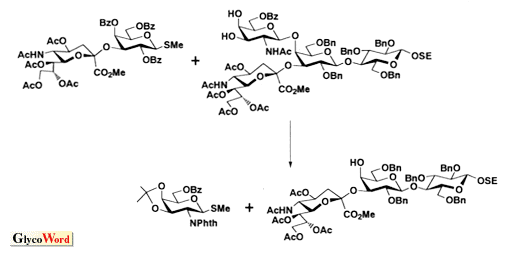
As shown in the retrosynthetic analysis of GD1a (Scheme), which can be applied to almost all gangliosides, the ceramide portion should be introduced after completing the construction of the sugar chain including sialic acid. When the ceramide is introduced into the glucose, which is the reducing terminal of the sugar chain, at the first stage of the synthetic route, the glucosyl ceramide obtained has too low reactivity to serve as a glycosyl acceptor for further elongation of the sugar chain because of the steric hindrance caused by the ceramide.
In addition, the following points must also be considered in order to accomplish glycosyl ceramide synthesis. First, azidosphingosine, a synthetic precursor of ceramide, serves as a much better glycosyl acceptor than the ceramide itself. Second, the protecting groups employed must be removed without affecting the functional groups of ceramide, especially the double bond. Therefore the benzyl group, one of the most popular protecting groups employed in carbohydrate chemistry, should be replaced with acyl groups before the coupling with ceramide. Third, the hydroxyl group at C-2 of the reducing terminal glucose should be protected with acyl groups, e.g. acetyl, benzoyl or pivaloyl group, to give beta-glycoside by a neighboring effect. And the more bulky group, pivaloyl>benzoyl>acetyl, affords the better yield by preventing ortho-ester formation, which is the cause of low yield.
On the other hand, sialic acid should be introduced to oligosaccharide at an early stage, as shown in the scheme. Because of the structural feature, sialic acid affords the corresponding glycoside in good yield only when glycosylated with reactive glycosyl acceptor, e.g. 6-O-benzoyl galactoside or 2,6,6'-tri-O-benzoyl lactoside. When reacted with less reactive acceptors, sialic acid is transformed into the 2,3-dehydro derivative, which is the major by-product of sialylation and lowers the yield. Therefore, sialyl galactoside or sialyl lactoside should be used as the building blocks for ganglioside synthesis. It is also important to note that the stereochemistry of the anomeric center of sialoside has been determined by the chemical shifts of NMR, e.g. H-3ax, H-3eq, H-4, by empirical rule. This means that the stereochemistry of a novel sialoside with complex oligosaccharides cannot be determined absolutely by NMR. This problem can be solved by employing an established building block. The synthetic strategy described can be applied for the synthesis of almost all gangliosides except the polysialo gangliosides, which require a different chemistry. |
|
|
 |
 |
 |
|
Fig. The structure of GD1a and its retrosynthetic analysis.
|
|
|
|
|
|
Hideharu Ishida (Department of Applied Bio-organic Chemistry, Gifu University) |
|
|
|
|
|
| References |
(1) |
T, Murase, H, Ishida, M, Kiso, A, Hasegawa Carbohydr. Res. 188, 71, 1989 |
|
(2) |
A, Kameyama, H, Ishida, M, Kiso, A, Hasegawa J. Carbohydr. Chem. 10, 549, 1991
|
|
(3) |
A, Hasegawa, HK, Ishida, T, Nagahama, M, Kiso J. Carbohydr. Chem. 12, 703, 1993 |
|
(4) |
H-K, Ishida, H, Ishida, M, Kiso, A, Hasegawa Tetrahedron: Asymmetry 5, 2493 (1994). |
|
(5) |
K, Hotta, H, Ishida, M, Kiso, A, Hasegawa J. Carbohydr. Chem. 14, 491 (1995). |
|
|
|
|
|
| Mar. 15, 2000 |
|
|
|
|
|
|
|



