Localization of glycosyltransferases | ||||||||||||||||||||||||||||||
 |
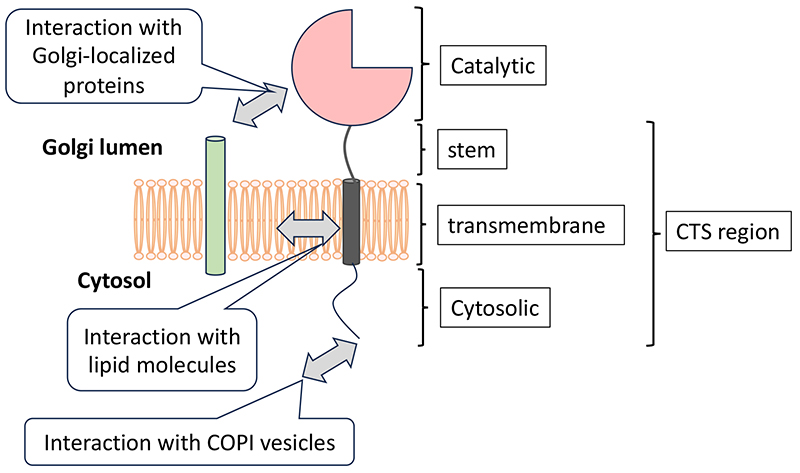
Glycosylation of proteins and lipids occurs stepwise and is formed by glycosyltransferases within the Golgi apparatus. For example, in N-glycosylation, a major type of protein glycosylation, the oligomannose type glycans attached to nascent glycoproteins in the endoplasmic reticulum (ER) are trimmed by mannosidases in the ER and Golgi apparatus. Subsequently, the glycan structures diversify through the action of glycosyltransferases adding N-acetylglucosamine, galactose, fucose, and sialic acid. This complex glycosylation process requires systematic localization and functioning of glycosyltransferases within the Golgi apparatus. For instance, mannosidases involved in the trimming of N-glycans localize to the cis-Golgi, while sialyltransferases, which add sialic acid to the non-reducing ends of glycans, are distributed in the trans-Golgi. The Golgi apparatus not only functions in the N-glycosylation on glycoproteins but also serves as a site for the glycosylation of lipids and the elongation of glycosaminoglycan (GAG) chains on proteoglycans. According to the previous paper (1), in Drosophila, proteins involved in these different glycosylation processes are localized to distinct Golgi stacks scattered throughout the cytoplasm. In contrast, in mammalian cells, the Golgi apparatus forms a single extensive structure known as the Golgi ribbon. Recent advances in electron microscopy have revealed that the structure of the Golgi apparatus is more complex than previously thought (2). It is assumed that glycosyltransferases are distributed in specific regions within this complex Golgi structure. For example, we have recently demonstrated that three types of sialyltransferases, previously thought to be located in the trans-Golgi, each has different distributions within the Golgi apparatus (3). Harada and his colleagues have shown that the Golgi apparatus is an organelle composing of small units that dynamically change through processes such as fragmentation, fusion, and deformation, with glycosyltransferases forming their own unique zones within these units (4). Glycosyltransferases in the Golgi apparatus are type II membrane proteins consisting of a catalytic domain, stem region, transmembrane region, and cytoplasmic domain (Figure). Additionally, some glycosyltransferases are known to have add-on domains adjacent to the catalytic domain (5). The localization of glycosyltransferases is determined by interactions with molecules within the Golgi apparatus. For instance, GlcNAc-1-phosphotransferase, responsible for synthesizing mannose-6-phosphate glycans involved in lysosomal transport, locates in the Golgi apparatus through interactions with the lysosomal enzyme trafficking factor (LYSET, TMEM251) (6,7). In LYSET-deficient cells, GlcNAc-1-phosphotransferase cannot reside in the Golgi apparatus, preventing mannose-6-phosphate glycan modification for lysosomal enzymes, giving rise to their mislocalization. Many glycosyltransferases do not exist as a monomer and are known to interact with transporters or other glycosyltransferases (see the "Regulation of glycosyltransferase by forming complexes" section). These interactions may regulate the localization of glycosyltransferases, though the specific regions responsible for the proven interactions remain limited.
The localization of membrane proteins is believed to be influenced by interactions with lipid molecules constituting the membrane. Electron microscopy images have shown that the thickness of membranes comprising the Golgi apparatus increases from the cis to the trans side (8). Bioinformatics analysis also indicates that glycosyltransferases in the trans-Golgi tend to have longer transmembrane regions compared to those in the ER or cis-Golgi (9). This suggests that the length of the transmembrane region, which stabilizes glycosyltransferases within the membrane, may correspond to the thickness of the lipid membrane, resulting in specific distributions. Identifying lipid molecules that co-localize with glycosyltransferases is crucial for elucidating the mechanisms of their localization, but analytical methods have not yet been established. The cytoplasmic regions of glycosyltransferases are known to interact with COPI coat subunits and COPI adaptors (e.g., GOLPH3/Vps74), retaining them within the Golgi apparatus (10). For instance, the cytoplasmic region of GALNT6 contains the sequence "MRLLRR," corresponding to the δ-COP binding consensus sequence ϕ-(K/R)-X-L-X-(K/R), and localizes to the cis-Golgi through direct interaction with δ-COP (11). ST3GAL4 and ST6GAL1 have been shown to interact with GOLPH3 through sequences (L-X-X-R/K) in their cytoplasmic regions (12). Recently, other COPI adaptors with functions similar to GOLPH3 have been discovered (13), highlighting the selective use of these molecules. However, glycosyltransferases with the same GOLPH3 binding motif can reside in either the cis or trans cisternae, indicating that the presence of the GOLPH3 binding motif alone cannot explain the localization of glycosyltransferases. Our research findings indicate that recombinant enzymes swapping the cytoplasmic, transmembrane, and stem (CTS) regions between B3GALT6 and B4GALT1 show higher co-localization with enzymes sharing the CTS region rather than with the original enzymes (3). However, even enzymes with common CTS regions do not entirely localize the same as the original enzymes, suggesting that the catalytic domain also influences localization. As discussed here, the localization of glycosyltransferases is likely determined by the combined effects of interactions with other molecules at their various regions such as the catalytic domain, stem region, transmembrane domain, and cytoplasmic region. While considerable knowledges have been accumulated on the individual localization controls of glycosyltransferases, comprehensive understanding requires extensive research targeting a broader range of enzymes. Hirokazu Yagi
|
|||||||||||||||||||||||||||||
| References | |
|---|---|
| (1) | Yano H, Yamamoto-Hino M, Abe M, Kuwahara R, Haraguchi S, Kusaka I, Awano W, Kinoshita-Toyoda A, Toyoda H, Goto S: Distinct functional units of the Golgi complex in Drosophila cells. Proc. Natl. Acad. Sci. U S A 102, 13467-13472, 2005 |
| (2) | Koga D, Kusumi S, Yagi H, Kato K: Three-dimensional analysis of the intracellular architecture by scanning electron microscopy. Microscopy 73, 215-225, 2024 |
| (3) | Yagi H, Tateo S, Saito T, Ohta Y, Nishi E, Obitsu S, Suzuki T, Seetaha S, Hellec C, Nakano A, Tojima T, Kato K: Deciphering the sub-Golgi localization of glycosyltransferases via 3D super-resolution imaging. Cell Struct. Funct. advpub, 24008, 2024 |
| (4) | Harada A, Kunii M, Kurokawa K, Sumi T, Kanda S, Zhang Y, Nadanaka S, Hirosawa KM, Tokunaga K, Tojima T, Taniguchi M, Moriwaki K, Yoshimura SI, Yamamoto-Hino M, Goto S, Katagiri T, Kume S, Hayashi-Nishino M, Nakano M, Miyoshi E, Suzuki KGN, Kitagawa H, Nakano A: Dynamic movement of the Golgi unit and its glycosylation enzyme zones. Nat. Commun. 15, 4514, 2024 |
| (5) | Yagi H, Takagi K, Kato K: Exploring Domain Architectures of Human Glycosyltransferases: Highlighting the Functional Diversity of Non-Catalytic Add-On Domains. Biochim. Biochim. Biophys. Acta Gen. Subj. in press. |
| (6) | Richards CM, Jabs S, Qiao W, Varanese LD, Schweizer M, Mosen PR, Riley NM, Klüssendorf M, Zengel JR, Flynn RA, Rustagi A, Widen JC, Peters CE, Ooi YS, Xie X, Shi PY, Bartenschlager R, Puschnik AS, Bogyo M, Bertozzi CR, Blish CA, Winter D, Nagamine CM, Braulke T, Carette JE: The human disease gene LYSET is essential for lysosomal enzyme transport and viral infection. Science 378, eabn5648, 2022 |
| (7) | Pechincha C, Groessl S, Kalis R, de Almeida M, Zanotti A, Wittmann M, Schneider M, de Campos RP, Rieser S, Brandstetter M, Schleiffer A, Müller-Decker K, Helm D, Jabs S, Haselbach D, Lemberg MK, Zuber J, Palm W: Lysosomal enzyme trafficking factor LYSET enables nutritional usage of extracellular proteins. Science 378, eabn5637, 2022 |
| (8) | Bykov YS, Schaffer M, Dodonova SO, Albert S, Plitzko JM, Baumeister W, Engel BD, Briggs JA: The structure of the COPI coat determined within the cell. Elife 6, e32493, 2017 |
| (9) | Sharpe HJ, Stevens TJ, Munro S: A comprehensive comparison of transmembrane domains reveals organelle-specific properties. Cell 142, 158-169, 2010 |
| (10) | Welch LG, Munro S: A tale of short tails, through thick and thin: investigating the sorting mechanisms of Golgi enzymes. FEBS Lett. 593, 2452-2465, 2019 |
| (11) | Liu L, Doray B, Kornfeld S: Recycling of Golgi glycosyltransferases requires direct binding to coatomer. Proc. Natl. Acad. Sci. U S A 115, 8984-8989, 2018 |
| (12) | Isaji T, Im S, Gu W, Wang Y, Hang Q, Lu J, Fukuda T, Hashii N, Takakura D, Kawasaki N, Miyoshi H, Gu J: An oncogenic protein Golgi phosphoprotein 3 up-regulates cell migration via sialylation. J. Biol. Chem. 289, 20694-20705, 2014 |
| (13) | Welch LG, Peak-Chew SY, Begum F, Stevens TJ, Munro S: GOLPH3 and GOLPH3L are broad-spectrum COPI adaptors for sorting into intra-Golgi transport vesicles. J. Cell Biol. 220, e202106115, 2021 |
Oct. 24, 2024