

 |
 |
Biosynthesis of Oligo/Polysialic Acid | |||||||||||||||||||||||||||||||||||
 | The oligo/polysialic acid (oligo/polySia) glycotope represents a group of oligo/polymers of Sia consisting of N-acetylneuraminic acid (Neu5Ac), N-glycolylneuraminic acid (Neu5Gc) and deaminoneuraminc acid (KDN; 2-keto-3-deoxy-D-glycero-D-galacto-nononic acid) (Fig. 1). The linkages between sialic acid residues are known to be alpha2->5O glycolyl, alpha2->8, alpha2->9, and alpha2->8/9. As shown in Table 1, many examples of the presence of oligo/polySia-containing glycoproteins have been demonstrated in eukaryotes, including recent findings of frequent occurrence of oligoSia structure on glycoproteins in mammals(1). Oligo/polySia structures on glycoproteins are widely recognized as regulator for cell-cell communication during development and cell differentiation based on various features such as stage- and tissue-specific expression, a large diversity in their structures, and unique physicochemical properties as polyanions (2). Biosynthesis of oligo/polySia is presumed to be complicated because of the large spectrum of their structures, which arises from the degree of polymerization (DP=2-100), variations in the (-ketosidic linkages to the penultimate Sia residues (alpha2->5O glycolyl, alpha2->8, and alpha2->9), and the composition of monomeric Sia (Neu5Ac, Neu5Gc, and KDN), which are often modified by O-acetylation, O-lactylation, O-sulfation, and lactonization). Here the biosynthesis of oligo/polySia chains of glycoproteins are described. Those for polySia (DP=100-200), which occurs on neuroinvasive bacterial capsular polysaccharide, and oligoSia (DP=2-4), which are often found in glycolipids, are not included. | ||||||||||||||||||||||||||||||||||
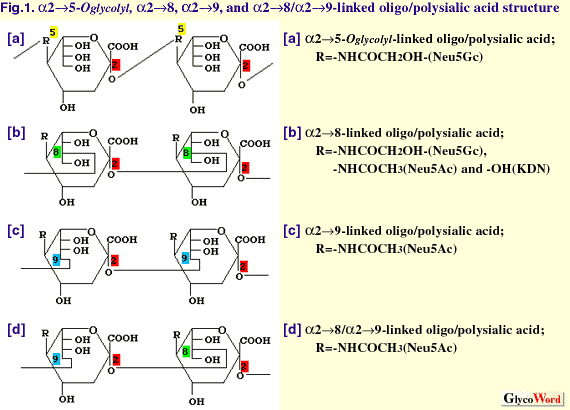
| |||||||||||||||||||||||||||||||||||
|
(a) Oligo/polysialic acid on O-linked glycoproteins The oligo/polySia chains on polysialoglycoproteins (PSGP) of rainbow trout have been demonstrated to be synthesized as follows (3). 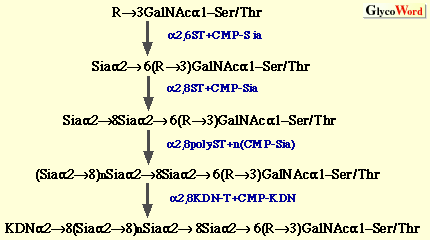 The oligo/polySia chains are synthesized by three enzymes, (2,6-sialyltransferase (alpha2,6ST), alpha2,8-sialyltransferase (alpha2,8ST, initiase), and alpha2,8-polysialyltranseferase (alpha2,8polyST, polymerase) in Golgi apparatus and/or cortical vesicles. The elongation of the chains is found to be terminated by introducing one KDN residue to the non-reducing terminal sialic acid residue in the alpha2->8-linkage. Enzymes involved in O-acetylation and O-lactylation of the Sia residues in PSGP have not yet been identified. The biosynthesis of alpha2->8-linked oligoKDN found in KDN-gp of rainbow trout ovarian fluid (4) and alpha2->5O glycolyl-linked oligo/polyNeu5Gc found in polySia-gp of sea urchin jelly coat (5) has not yet been studied. Interestingly, in the latter case, 9-O-sulfation of non-reducing terminal Neu5Gc residue is suggested to play a role as the termination signal. (b) Oligo/polysialic acid on N-linked glycoproteins The elucidation of biosynthesis of polySia on neural cell adhesion molecule (N-CAM) has made big advances by the identification and cloning of two alpha2,8-sialyltransferases (Table II), from mouse (6), Chinese hamster (7), and human (8). The present understanding of the pathways for oligo/polysialylation on N-linked glycan chains is summarized as follows. 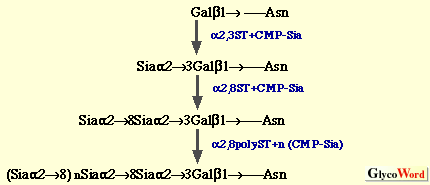 The alpha2,8-polysialyltransferase(s) (alpha2,8polyST) which catalyzes elongation reaction is considered to be ST8Sia II (STX) and/or ST8Sia IV (PST). However, it still remains to be clarified if the alpha2,8ST which initiates formation of oligo/polySia is a different enzyme from alpha2,8polySTs. Recently, not only expression of the enzymes ST8Sia II and ST8Sia IV, but also intracellular Ca2+ has been shown to control the biosynthesis of polySia on N-CAM (9). Structural analysis of carbohydrate chains derived from embryonic N-CAM indicates that O-acetylation of polySia may be related to the termination of elongation (10). The biosynthetic pathway for oligoSia chains on glycoproteins other than N-CAM is unknown; however, ST8Sia III (Table 2) catalyzing formation of dimer of alpha2->8linked Sia on N-linked glycoproteins may possibly be involved. Elucidation of mechanisms for biosynthesis of other types of oligo/polySia chains, such as alpha2->5O glycolyl linked oligo/polyNeu5Gc and alpha2->9 linked oligo/polySia, modifications of sialic acid residues, and termination of elongation of oligo/polySia chains is interesting future work on biosynthesis of oligo/polySia. | |||||||||||||||||||||||||||||||||||
| Chihiro Sato (Nagoya University) Ken Kitajima (Nagoya University Bioscience Center) | |||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||
| Apr. 15, 2001 | |||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||