

 |
 |
Glycolipid Microdomain |
 | |||||||||||||
| (First version published: Jun.15, 1998) | ||||||||||||||
 | Glycosphingolipids (GSLs) are relatively rich in saturated fatty acyl chains while glycerophospholipids are rich in cis-unsaturated fatty acyl chains. GSLs form domain structures as a result of phase-separation in the lipid membrane. The glyceroglycolipid, phosphatidylglucoside, which has stearic acid (C18:0) on the C1 site and arachidic acid (C20:0) on the C2 site, forms domains in HL60 cell membrane. Condensation of signaling molecules into such microdomains increases frequency of meeting of membrane molecules and efficiency of transmembrane signal transductions. | |||||||||||||
 |
||||||||||||||
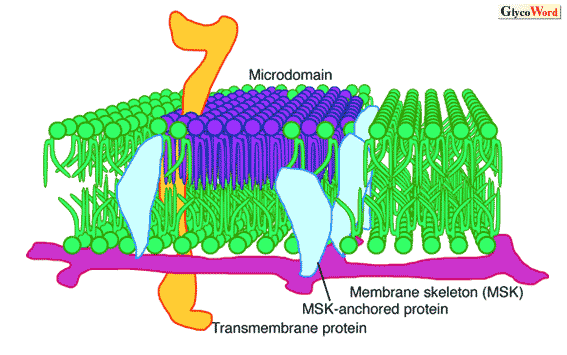
| Figure Membrane lipids and their microdomains of which movement is restricted by "Picket" of membrane skeleton-anchored proteins | ||||||||||||||
|
Microdomains termed raft or DIG are rich in cholesterol but other domains are found to contain little if any cholesterol. Hakomori and colleagues termed such ganglioside-enriched domains"glycosynapses"that mediate cell adhesion and cell-cell interaction. In glycosynapses, tetraspanins such as CD9 and CD81, integrin receptors, and cell growth factor receptors gather and modulate cellular phenotype by regulating signals of integrins and growth factors. Like raft, Src family kinases and small G-proteins gather in the cytosolic side of the membrane domain. Transmembrane molecules such as growth factor receptors and tetraspanins may coordinate the gathering of these signaling molecules on both sides of the membrane. At physiological temperature, lipid molecules, which construct biomembranes, usually move around and membrane molecules floating on the membrane also move freely. Thus, microdomains such as raft and glycosynapse are always moving and changing. The movement is restricted by the inner membrane mesh structures of cytoskeletal actins, "membrane skeleton." The mesh size is 30-230 nm (depending on the cell type). In the membrane compartment, membrane proteins and lipids move freely by thermal motion but the movement of transmembrane proteins is limited by membrane skeletons (fence model). Membrane proteins anchored to the membrane skeleton limit leakage of lipids from the compartment (picket model). Thus, membrane molecules stay for a while in one compartment then move to the next compartment. Microdomains are formed in compartments. Membrane compartments affect signal transduction at microdomains. Positional information of the signal may be given by the transient binding of the ligand-bound receptor to the membrane skeleton. |
||||||||||||||
| Hideyoshi Higashi (Division of Glyco-signal Research, Tohoku Pharmaceutical University) | ||||||||||||||
| ||||||||||||||
| Feb.01, 2007 | ||||||||||||||
| ||||||||||||||