

 |
 |
Distribution of Glycosphingolipids in the Cell |
 | |||||||||||||
| (Update Issue: Feb.1, 2007) | ||||||||||||||
 |
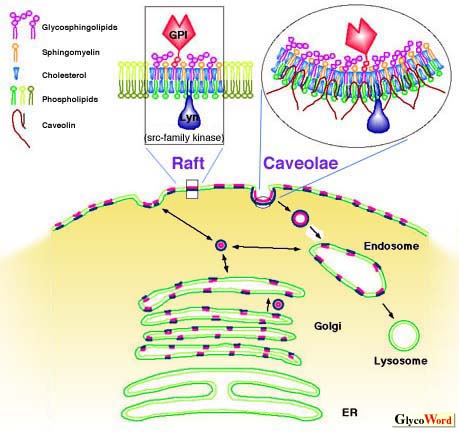
Most glycosphingolipids (GSLs) are distributed in membranous structures in the cell. While their density in plasma membrane is high, two-thirds of the total GSLs is distributed in intracellular membrane such as Golgi apparatus, endosomes, lysosomes, nuclear membrane, ER, and mitochondria. GSLs, along with other membrane components, circulate through these organelles. In Golgi apparatus, GSLs are newly synthesized by the addition of saccharides one by one, and in lysosomes, they are degraded by the removal of saccharides one by one. GSLs, which are incorporated by endocytosis, are sorted into lysosomes or Golgi apparatus from endosomes. Newly synthesized GSLs are sorted into plasma membranes through endosomes. A potion of endocytosed GSLs are returned back to the plasma membrane. One half of GSLs in plasma membrane were recycled for 30 to 40 min although the time varied cell to cell, and their composition in the plasma membrane remains constant. Most GSLs are transported between membranes as small vesicles maintaining a bilayer structure. In plasma membrane, GSLs form clusters, called rafts, with cholesterol and relatively less phospholipids than other areas of plasma membrane. There are receptors for intercellular signal transducers such as GPI-anchored proteins on the exoplasmic face of the rafts and src family kinases on the cytosolic face. Thus they play a role in transmembrane signal transduction. Small vesicles containing rafts move among Golgi complexes, plasma membrane, and endosomes. Specific annexins may be involved in vesicular docking and fusion. Rafts are extracted in low density fractions, insoluble in 1% Triton 100 at 4oC, these are called detergent-insoluble glycolipid-enriched complexes, or detergent insoluble glycosphingolipid-enriched domains (DIGs). Into this fraction, micro invaginated structures (diameter, around 50 nm) of the plasma membrane, caveolae, are also extracted. Caveolae also consist of GSLs, cholesterol, GPI-anchored proteins, src family kinases, trimeric G proteins, Ras, and a caveolae-specific membrane protein, caveolin, which associate themselves and bind to cholesterol and may take the invaginated shape. Caveolae are domains where endocytosis can occur. Rafts may be trapped in caveolae and the receptor molecules are clustered more densely. This may improve efficiency of signal transduction. GSLs interact with receptor molecules and kinases, and modulate their functions. GSLs are distributed in exoplasmic leaflets of plasma membrane and the lumenal side of organelles. However, the first step of the biosynthesis of most GSLs, i.e. transfer of a glucosyl residue to ceramide, occurs at the cytosolic surface of cis Golgi and the other sugars are transferred to the lumenal face of the Golgi complex. In cells with polarity, e.g. epithelial cells in intestine and kidney, GSLs are sorted to their proper side, apical or basolateral surface, thus the composition of GSLs differs at the apical and basolateral side. Moreover, exoplasmic components of the plasma membrane cannot move freely across tight junctions, which separate the apical and basolateral membrane, thus the GSL composition of each side remains unchanged. Some GSLs may be distributed in cytosol. The soluble fraction of brain contains 5% of the total gangliosides and their composition is similar to that of the membrane fraction. Some GSLs associate with vimentin, a component of intracellular intermediate filaments, and this is one of the routes of GSL recycling. There are cytosolic proteins which transfer GSLs between one membrane and another. | |||||||||||||
 | ||||||||||||||
| Hideyoshi Higashi (Mitsubishi Kasei Institute of Life Sciences) | ||||||||||||||
| ||||||||||||||
| Jun.15, 1998 | ||||||||||||||
| ||||||||||||||