

 |
 |
Acceleration of Sialyl Lewisx/a Expression Associated with Cancer Progression |
||||||||||||||||||||||||||||||||||||||
 |
Cell surface carbohydrate determinants undergo drastic changes during malignant transformation. It is well known that expression of sialyl Lewisx and sialyl Lewisa determinants is markedly enhanced in cancer cells. These determinants serve as ligands for selectins, the cell adhesion molecules present on endothelial cells, and they also mediate hematogenous metastasis of cancers. Normal epithelial cells express various carbohydrate determinants,
some of which have structures more complex than that of sialyl Lewisx/a.
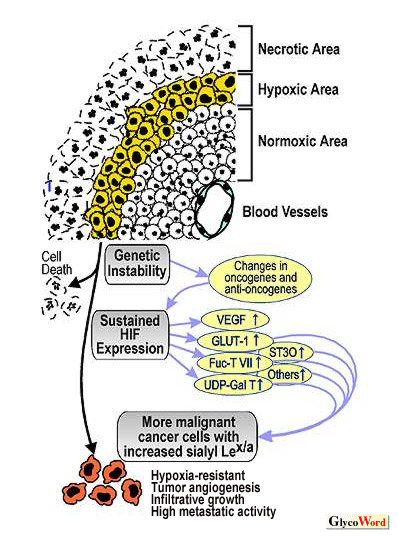
Good examples are sialyl 6-sulfo Lewisx and 2 Progression of cancer is a long process, sometimes spanning a number of years. Later, in the more advanced stages, cancer cells accumulate genetic abnormality and more malignant cancer cells evolve according to the principle of survival of the fittest, and such cells have higher infiltrative and metastatic activities. Expression of sialyl Lewisx/a determinants is further accelerated during the course of cancer progression. One of the selection mechanisms for more malignant cells during cancer
progression is their resistance adaptation against hypoxic conditions.
Because of the uncontrolled proliferation of cancer cells, delivery
of oxygen is significantly reduced in solid tumors, and some of the
cancer cells are always subjected to a hypoxic environment (Fig.
1). When the normal cells are exposed to such an environment,
the One of the consequences of sustained HIF expression is a particular deviation in the intracellular carbohydrate metabolism, a metabolic shift from oxidative to elevated anaerobic glycolysis (the Warburg effect), which is correlated with the increased gene expression of some glycolytic enzymes and glucose transporters including GLUT1. Another consequence is the facilitated production of vascular endothelial growth factor (VEGF), which supports tumor angiogenesis. |
|||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
Recently, we found a significant induction of sialyl Lewisx and sialyl Lewisa expression on epithelial cells cultured under hypoxic conditions, together with a concomitant increase of E-selectin binding activity (3). Participation of HIF in the process, direct or indirect, is clear since the introduction of dominant negative HIF to the cells completely abrogated these induced effects. A significant induction of transcription of genes for GLUT1, UDP-galactose transporter (UGT1), a fucosyltransferase (FUT7), a sialyltransferase (ST3O) and some other genes closely related to the carbohydrate metabolism was detected after hypoxic culture by DNA microarray technique and RT-PCR (3). These results strongly suggest that augmentation of sialyl Lewisx/a expression on cancer cells is intimately related to the process of cancer progression, and the more malignant cancer cells tend to have enhanced expression of these carbohydrate determinants. This mechanism seems to contribute to the further enhancement of sialyl Lewisx/a expression on advanced cancer cells, which had already been predisposed to express these determinants by epigenetic gene silencing in the early stages of carcinogenesis. Expression of genes for GLUT1, UGT1, FUT7, and ST3O is significantly elevated in cancer cells prepared from surgical specimens of colon cancers, compared to non-malignant colonic epithelial cells taken from the same patients, especially those in the advanced stages such as Dukesí C and D (3, 4). This suggests that the HIF-induced transcription of these genes indeed takes place in the actual cancers of patients. Interaction of cancer cells with endothelial cells mediated by selectins and sialyl Lewisx/a determinants may have patho-physiological relevance not only in the hematogenous metastasis of cancer as formulated earlier but also in the blood-vessel formation of tumors. We assessed the possible significance of this cell adhesion system in tumor vascularization in a recent study by employing an in vivo model using a cultured endothelial cell line, which expressed selectins and could adhere to human cancer cells expressing sialyl Lewisx/a (5). When human cancer cells and the endothelial cell line cells at a ratio of 10:1 were subcutaneously co-transplanted into the back of nude rats, the tumors formed were extensively vascularized throughout by the blood vessel-like structures formed by the endothelial cells, the lumen of which contained the host blood cells. The administration of anti-Lewisx/a antibodies resulted in a marked reduction in the size of tumors which were not vascularized and were accompanied by independent tiny remnant clumps composed of endothelial cells. These results served to corroborate the hypothesis that cell adhesion mediated by selectins and sialyl Lewisa/x determinants is significantly involved in tumor vascularization. Cancer cells try to survive hypoxic conditions by acquiring sustained expression of HIF, thus altering their glucose metabolic pathway from aerobic to anaerobic, and also by inducing endothelial cell growth with VEGF. The HIF-induced enhancement of sialyl Lewisx/a expression on cancer also seems to be a link in the chain of these unfolding events, given that sialyl Lewisx/a determinants promote tumor vascularization (6). |
||||||||||||||||||||||||||||||||||||||
| Reiji Kannagi (Molecular Pathology, Aichi Cancer Center) | ||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Dec. 28, 2004 | ||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||