

 |
 |
Nucleotide Sugar Transporters and Synthesis of Glycoconjugates |
 |
|||||||||||||||||||||||||
 |
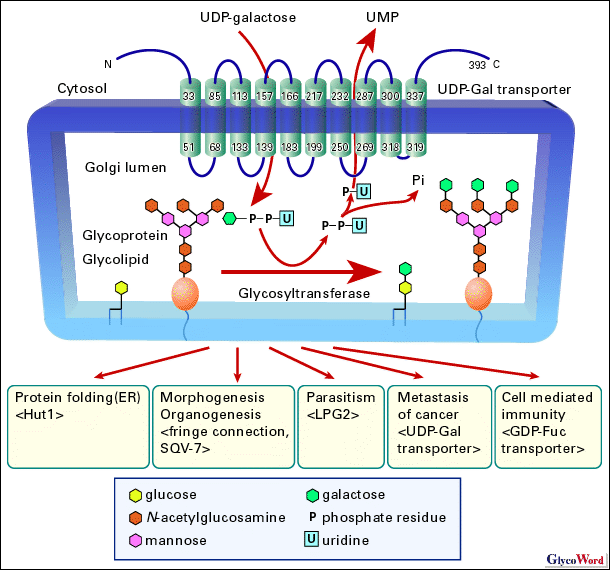
The carbohydrate chain is elongated by glycosyltransferases adding monosaccharides at their ends where they utilize nucleotide sugars for sugar donors activated by conjunction of nucleotides. Nucleotide sugars are synthesized in cytosol, except for CMP-sialic acid, which is synthesized in nucleus, while most sugar addition takes place within Golgi apparatus or endoplasmic reticulum (ER) lumen separated from cytosol by membrane. Nucleotide sugar transporters must translocate the sugar donors from cytosol into lumen of the cell organelles across the membrane, since nucleotide sugars cannot permeate the membrane bilayer. Nucleotide sugar transporters are very hydrophobic proteins ranging from 340 a. a. to 400 a. a. long. They reside in Golgi apparatus and/or ER membrane exposing their C- and N-terminal regions to cytosol, and are highly likely to have ten transmembrane helices. They antiport nucleotide sugars pooled in cytosol into lumen of Golgi apparatus and/or ER lumen with the corresponding nucleoside monophosphates such as UMP for UDP-sugars, GMP for GDP-sugars, and CMP for CMP-sugars. The transported nucleotide sugars are utilized as sugar donors by glycosyltransferases for synthesis of sugar chains of glycoproteins, glycolipids and polysaccharides. UDP-glucuronic acid also serves as a substrate for glucuronidation in ER lumen. There are many molecular species of nucleotide sugars, and it is assumed that there is a specific nucleotide sugar transporter for each nucleotide sugar (1-3). Several pieces of evidence indicate that nucleotide sugar transporter assembles into a homodimer. It is further suggested that the homodimer formation is required to transport a nucleotide sugar from complementation analysis of deficient cells introducing simultaneously active and inactive GDP-Man transporters. It is also known that N- and C-terminal cytosolic regions of the transporter can be deleted without affecting its cellular localization and transporting activity. A recent report demonstrated that UDP-Gal transporter obtains additional CMP-Sia transporting ability by replacement with portions of CMP-Sia transporter. Inversely, CMP-Sia transporter acquires additional UDP-Gal transporting activity by replacement with other portions of UDP-Gal transporter (for review see ref. 3). Development proceeds under molecular control by precise regulation of growth factor signaling. One such regulation is the modification of Drosophila Notch receptor with the sugar chains coupled with a nucleotide sugar transporter, i.e. fringe connection (4). Notch receptor is involved in patterning the body plan such as determination of the dorsal-ventral boundary in the wing and eye imaginal discs during the development. Fringe, a fucose-specific beta1,3-N-acetylglucosamine transferase, modifies the Notch receptor elongating the O-linked fucose residues on the receptor that modulates the interaction of the Notch receptor with its ligands, Delta and Serrate. The difference in sensitivity of Notch receptor to those ligands ensures that the Notch receptor is activated only at the boundaries of the Fringe and ligand expression and eventually forms the dorsal-ventral boundary. A disruption of the fringe connection gene resulted in a nicked wing margin, thickened wing vein, rough eye, duplicated notal macrochaetae and fused leg segments due to aberrant boundary formations. In Caenorhabditis elegans, a nucleotide sugar transporter SQV-7 has been shown to be involved in the invagination of epithelial vulva, while its role in the regulation of the organogenesis is still unknown. A recent study (5) suggests that Hut1 is involved in the protein folding process in the ER lumen. The Hut1 localizes at the ER, and the transporters from Schizosaccharomyces pombe and A. thaliana both transport UDP-Gal and UDP-Glu. Yeast cells with a disrupted hut1 gene proved to be one of the phenotypes observed in deficient mutants in the ER quality control of proteins. Advanced colon cancer cells express the carbohydrate determinants, sialyl Lewis A/X, which binds to E-selectin that is a cell adhesion molecule expressed on vascular endothelial cells. The binding between the ligands and the selectin results in the cell adhesion to vascular endothelium followed by the extravasation of the cancer cells. They then form a new metastatic lesion in the connective tissues. The expression of sialyl Lewis A/X on the cell surface thus contributes to hematogenous metastasis of the cancer cells. It was observed that the amount of mRNA for UDP-Gal transporter was significantly increased in malignant colonic cancer tissues (6). The expression of sialyl Lewis A/X determinants increased markedly, when the expression of UDP-Gal transporter was enhanced with transfection of an expression vector of the transporter to cultured human colon cancer cell lines. The transfected cells showed enhancement of cell adhesion to cells expressing vascular E-selectin. Those findings suggest that UDP-Gal transporter is involved in hematogenous metastasis. It has been shown that GDP-Fuc transporter is involved in cellular immunity. Leukocyte adhesion deficiency type II (LAD II/CDG IIc) is one of the congenital disorders of glycosylation, characterized by the lack of fucosylated glycoconjugates owing to a defect in the GDP-Fuc transporter. The facial expression of patients with LAD II/CDG-IIc was distinctive and characterized by a flat face with a broad nasal tip and puffy eyelids. They exhibited severe psychomotor, growth and mental retardation, and suffered from recurrent infections. A set of fucose-containing surface determinants, sialyl Lewis X, is known to be an important ligand for selectins in the initial interaction of leukocyte to endothelium followed by leukocyte rolling. The single amino acid changes of GDP-Fuc transporter from the patients greatly reduced or inactivated the transporting activity that leads to the loss of fucosylated glycoconjugates including sialyl Lewis X on leukocyte surface, causing immunodeficiency (8). Molecular identities of nucleotide sugar transporters were unveiled by cloning of K. lactis UDP-GlcNAc transporter, murine CMP-Sia transporter, and human UDP-Gal transporter in 1996 (1-3). That enabled us to carry out studies using recombinant or modified transporter proteins, analysis of genes from mutants, introduction and disruption of genes of nucleotide sugar transporters. Those investigations will reveal the relationship between transporting function and structure, physiological role of nucleotide transporters and their involvement in disorders in detail. |
|||||||||||||||||||||||||
 |
||||||||||||||||||||||||||
| Nobuhiro Ishida (Tokyo Metropolitan Institute of Medical Science) |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Nov. 28, 2002 | ||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||