 |
Starch synthase (SS; EC2.4.1.21) is one of key enzymes in the synthesis of starch by obtained elongating 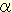 1,4-glucosidic linkages of amylopectin and amylose. Higher plants have several SS isozymes or isoforms; for example, GBSSI, SSI, SSIIa, SSIIb, and SSIII are present in maize (Table 1). Comparison of these isozymes reveals varied size in N-terminal extensions with very little sequence similarity while there are distinct consensus sequences in the C terminal region among them. GBSSI, a starch granule-bound starch synthase, is most widely examined enzyme in many plants. Lesion of this enzyme by mutation results in elimination of amylose from the starch granule, indicating that GBSSI is related to amylose biosynthesis. The other soluble isozymes which are involved in amylopectin biosynthesis have been referred to as SSS (soluble starch synthase). However, SSI and SSII are also associated with starch granules. Thus, these isozymes are usually called merely 'SS'. The studies with some mutants or transformants of individual SS isoforms have given us important clues in understanding their contribution to the structure of amylopectin. 1,4-glucosidic linkages of amylopectin and amylose. Higher plants have several SS isozymes or isoforms; for example, GBSSI, SSI, SSIIa, SSIIb, and SSIII are present in maize (Table 1). Comparison of these isozymes reveals varied size in N-terminal extensions with very little sequence similarity while there are distinct consensus sequences in the C terminal region among them. GBSSI, a starch granule-bound starch synthase, is most widely examined enzyme in many plants. Lesion of this enzyme by mutation results in elimination of amylose from the starch granule, indicating that GBSSI is related to amylose biosynthesis. The other soluble isozymes which are involved in amylopectin biosynthesis have been referred to as SSS (soluble starch synthase). However, SSI and SSII are also associated with starch granules. Thus, these isozymes are usually called merely 'SS'. The studies with some mutants or transformants of individual SS isoforms have given us important clues in understanding their contribution to the structure of amylopectin.
|
|
|
Recently, we isolated the SSI mutant in rice using the transposon (Tos17) tagging method. 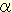 1,4-Glucan chains with DP8-12 in the debranched amylopectin of the mutant are reduced as compared with the wild-type amylopectin, although the weight of the mutant seed is unchanged. This suggests that SSI is involved in the synthesis of those short chains in rice endosperm. 1,4-Glucan chains with DP8-12 in the debranched amylopectin of the mutant are reduced as compared with the wild-type amylopectin, although the weight of the mutant seed is unchanged. This suggests that SSI is involved in the synthesis of those short chains in rice endosperm.
The pea mutant referred to as rugosus-5 is deficient in SSII. The average length of the A chains of the mutant amylopectin is reduced. The ratio of A and B1 chains to longer B chains is twice as large in rugosus-5 amylopectin as in wild-type amylopectin, suggesting that SSII plays a specific role in the synthesis of B2 and B3 chains of amylopectin in pea embryo(1). On the other hand, amylopectin chains of 12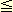 DP DP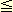 21 are significantly depleted in the antisense-SSII transformed potato(2). The same trend is observed in Japonica rice as compared with Indica rice, and SSIIa is considered to be lacking or lower in Japonica rice as compared with Indica rice(3). These results suggest that SSII in these plants is responsible for the synthesis of A and B1 chains. 21 are significantly depleted in the antisense-SSII transformed potato(2). The same trend is observed in Japonica rice as compared with Indica rice, and SSIIa is considered to be lacking or lower in Japonica rice as compared with Indica rice(3). These results suggest that SSII in these plants is responsible for the synthesis of A and B1 chains.
dull-1 in maize, whose mature kernels are somewhat dull in appearance, is mutated in SSIII gene. Total carbohydrate content in mature dull-1 mutant kernel is slightly lower than wild-type whereas the apparent amylose content from the dull-1 mutant is higher. Moreover, approximately 15% of the starch in the dull-1 mutant endosperms is known to be present as an 'intermediate polyglucan' which is highly branched(4). In antisense-SSIII transformed potato, B1-B2 chains of amylopectin are reduced, while A+B1 chains are higher as compared with the wild type and it has starch granules with strikingly altered morphology and gelatinization temperature(2). These results show that SSIII is related to the synthesis of B chain of amylopectin in these plants.
In an attempt to analyze the function of SS isozymes, Guan and Keeling(5) expressed various combinations of maize SS isozyme and maize BE genes in E. coli. While maize SSI preferentially synthesizes short chains (6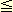 DP DP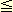 15), SSIIa and SSIIb preferentially synthesize longer chains (DP>24) and intermediate chains (16 15), SSIIa and SSIIb preferentially synthesize longer chains (DP>24) and intermediate chains (16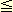 DP DP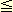 24), respectively. These results are consistent with those of SSI mutant in rice and the rugosus-5 mutant described above. 24), respectively. These results are consistent with those of SSI mutant in rice and the rugosus-5 mutant described above.
It is highly possible that some SS isozymes are associated with BE isozymes when they synthesize amylopectin molecules(4). Further studies on gene expression in vitro, improvement of methods for analysis of the amylopectin fine structure and isolation of mutants or transformants are needed to understand the distinct roles of individual SS isozymes. |
|
|
| References |
(1) |
Craig J, Lloyd JR, Tomlinsom K, Barber L, Edwards A, Wang TL, Martin C, Hendley CL, Smith AM, Plant Cell. 10, 413 |
|
(2) |
Edwards A, Fulton DC, Hylton CM, Jobling SA, Gidley M, Rossner U, Martin C, Smith AM, Plant J, 17, 251 |
|
(3) |
Umemoto, T., Yano M, Satoh H, Shomura A, Nakamura Y, Theor. Appl. Genet, 104, 1 |
|
(4) |
Gao M, Wanat J, Stinard PS, James MG, Myers AM, Plant Cell. 10, 399, 1998 |
|
(5) |
Guan HP, Keeling PL, Trends in Glycosci. Glycotech. 10, 307, 1998 |
|
|
|
|
|



