 |
Stem cells are undifferentiated cells that have differentiation ability to differentiate into various somatic cells and self-renewal ability to divide while maintaining their properties. There are various types of stem cell, including embryonic stem (ES) cells established from the inner cell mass of early embryos and induced pluripotent stem (iPS) cells established by reprogramming from somatic cells, which are collectively called “pluripotent stem cells”; tissue stem cells involved in forming and maintaining organs such as the brain, blood, and liver; and cancer stem cells, which have the characteristics of stem cells in cancer tissue and may cause recurrence and metastasis. Thus, stem cell study is a research field with applications not only in developmental biology but also in regenerative medicine and cancer treatment. In order to maintain their characteristics, stem cells must be able to receive signals from outside the cell and transduce them within the cell. In particular, the stem cell “niche” — that is, the microenvironment around the stem cells — plays an important role in maintaining their properties. A key component of the stem cell niche is the extracellular matrix (ECM), which in turns contains glycosaminoglycans (GAGs) as major constituents. GAGs comprise a large class of polysaccharides with specific repeating disaccharides, and include heparan sulfate (HS), chondroitin sulfate (CS), dermatan sulfate (DS), keratan sulfate (KS), and hyaluronan (HA). These structures have been described in detail in other papers. In this article, we focus on the function of GAGs in pluripotent stem cells.
Role of HS in pluripotent stem cells
Sulfated GAGs including HS mainly bind via their sulfate groups to morphogens such as bone morphogenetic protein (BMP) and Wnt, and growth factors such as fibroblast growth factor (FGF) and epidermal growth factor (EGF); they act as co-receptors, as well as stabilizing these factors in various cells. Indeed, mouse ES cells harboring short-chain HS caused by treatment with heparitinase (microbial-derived HS-degrading enzyme) and knockdown of Ext1, which encodes a glycosyltransferase that synthesizes HS chains, show low levels of Wnt3a and BMP4 signaling activity and lose their undifferentiated state (1). Similar results have been reported by knockdown of Ndst1 or Ndst2, which encodes N-deacetylase/N-sulfotransferase (NDST) that is involved in N-deacetylation and N-sulfation of HS, indicating that HS sulfation is important for maintaining the undifferentiated state of mouse ES cells (1). Furthermore, Ext1-deficient mouse ES cells (i.e., HS-deficient cells) show loss of FGF4 signaling, which induces differentiation, resulting in a phenotype that cannot differentiate (1). This phenotype can be rescued by adding HS. In addition, expression of the HS 3-O-sulfotransferase HS3ST5 and sulfation at C-3 position of N-acetylglucosamine (GlcNAc) (3-O-HS) increases during mouse ES cell differentiation (1). 3-O-HS contributes to the disruption of mouse ES cell undifferentiation by activating Fas signaling and inducing Nanog protein degradation via the caspase pathway. Collectively, these findings reveal that HS regulates an undifferentiated state of mouse ES cells through its presence, length, and level of sulfation (Figure 1). In the future, elucidation of the function of individual sulfated modification of HS and control of stem cells will be necessary for the realization of regenerative medicine and cell therapy.
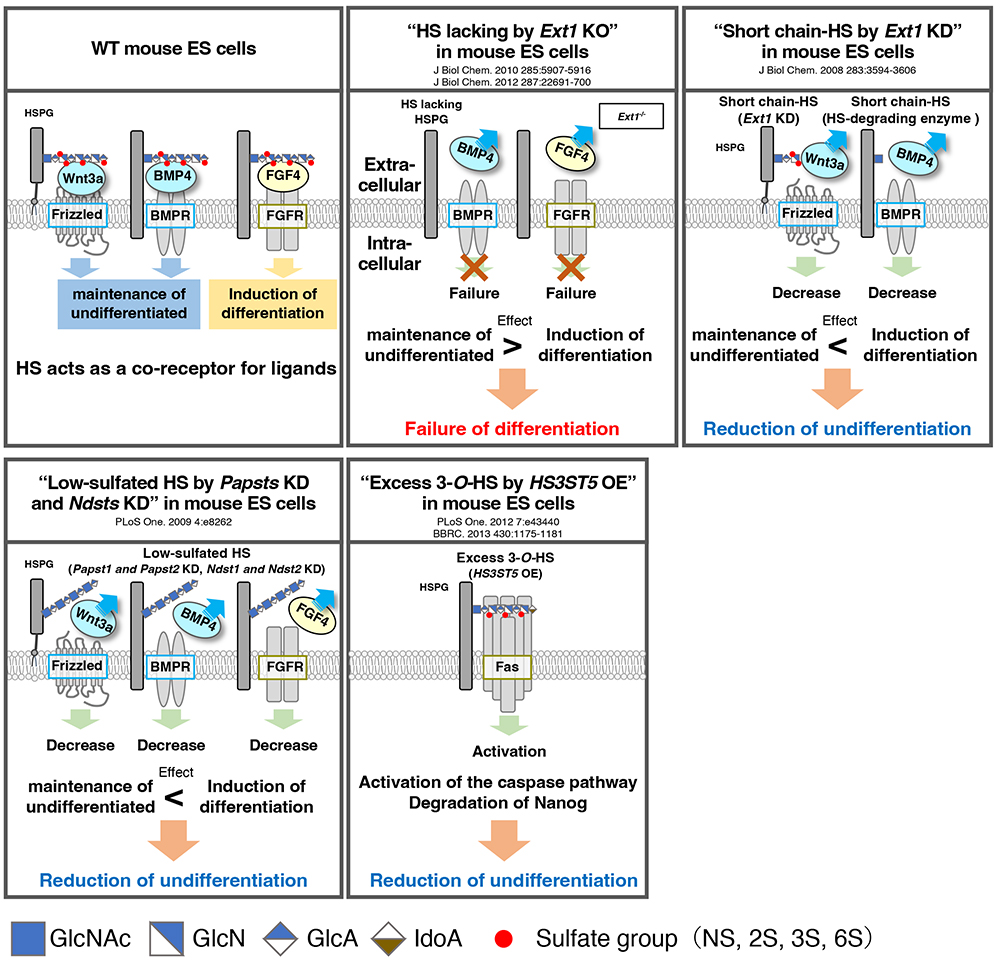
Figure 1. Role of HS in pluripotent stem cells
Scheme shows the function of HS reported from various studies. The presence, length, and sulfation status of HS have different effects on cell fate.
KD: knockdown by RNAi; HSPG, heparan sulfate proteoglycan; Papst, PAPS (3′-phosphoadenosine 5′-phosphosulfate) transporter; Ndst, N-deacetylase/N-sulfotransferase; HS3ST5, heparan sulfate 3-O-sulfotransferase 5; BMP4, bone morphogenetic protein 4; BMPR, bone formation protein receptors; FGF4, fibroblast growth factor 4; FGFR, fibroblast growth factor receptor.
|
Role of CS/DS in pluripotent stem cells
The function of CS in mouse ES cells has been evaluated using Glucuronyltransferase I (GlcAT-I)-deficient cells (2). GlcAT-1-/- cells do not differentiate even under differentiation-inducing conditions and maintain their undifferentiated state, but this phenotype can be rescued by the addition of CS-A (CS-4-O-sulfate) and CS-E (CS-4-O-, 6-O-disulfate). Furthermore, both CS-A and CS-E activate downstream RhoA/Rock signaling by binding to E-cadherin, which contributes to the induction of mouse ES cells differentiation (Figure 2).
Analysis of the function of DS, which has an iduronic acid (IdoA) group epimerized from glucuronic acid (GlcA) on the CS chain and is synthesized by Dermatan-4-O-sulfotransferase 1 (D4ST1), has focused on D4ST1 in mouse ES cells (3). Decreased expression of D4ST1 in mouse ES cells leads to a decrease in undifferentiated state and differentiation into endoderm. In addition, Wnt signaling, which is also involved in endoderm differentiation, is activated by decreased D4ST1 expression, while BMP signaling, which maintains an undifferentiated state, is suppressed (3). These observations indicate that DS contributes to maintaining the undifferentiated state of mouse ES cells through Wnt and BMP signaling (Figure 2). Moreover, it has been found that the expression of D4ST1 increases with neural differentiation from mouse ES cells, which suggests that DS expression is involved in the differentiation of neurons (4). Indeed, addition of DS to culture medium promotes both neuronal differentiation and neurite extension, and also enhances Erk1/2 signaling (Figure 2). Interestingly, neural stem cells derived from human fetal cerebral cortex also shows enhanced neuronal differentiation and accelerated neuronal migration on the addition of DS. Much research on CS/DS has been reported in recent years; in particular, accumulated knowledge of the functions of CD/DS in the nervous system has progressed and is expected to contribute to the treatment of neurological diseases in the future.
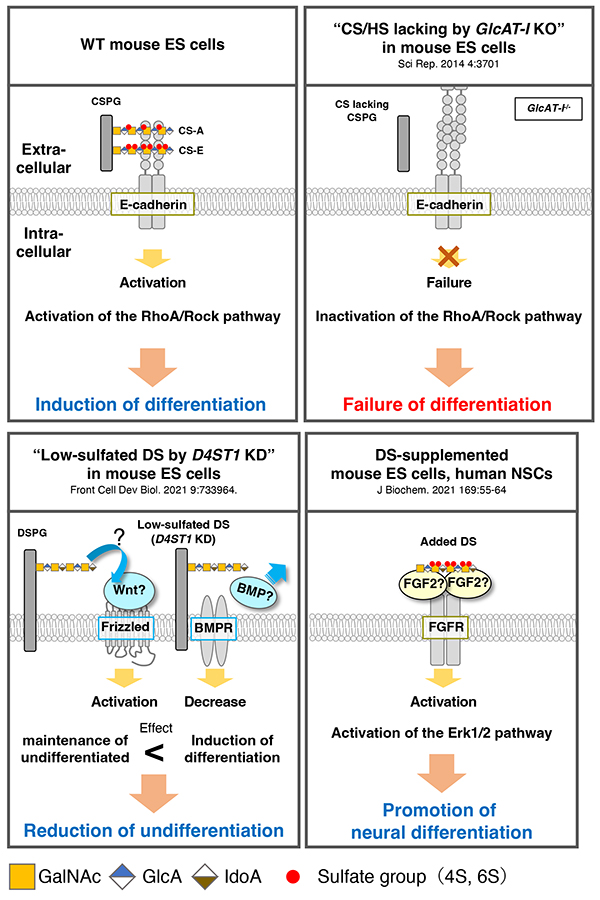
Figure 2. Role of CS/DS in pluripotent stem cells
Scheme shows the function of CS/DS reported from various studies. CS and DS trigger different signaling transduction pathways.
KD: knockdown by RNAi; CSPG, chondroitin sulfate proteoglycan; DSPG, dermatan sulfate proteoglycan; CS-A, chondroitin sulfate-A (4-O-sulfate); CS-E, chondroitin sulfate-E (4-O-, 6-O-disulfate); FGF, fibroblast growth factor 2; FGFR, fibroblast growth factor receptor.
|
Role of KS in pluripotent stem cells
Little is known about the role of KS, but it may be useful as a biomarker for human pluripotent stem cells. Recently, R-10G that is mouse antibody specifically recognizing low-sulfated KS on human pluripotent stem cells has been developed (5). It has been shown that the epitope is formed by KS attached to the Podocalyxin protein. So far, however, the function of KS in pluripotent stem cells has not been elucidated, and further research is expected.
Role of HA in pluripotent stem cells
HA has been well studied as a scaffold for pluripotent stem cells. The findings have been applied to the development of HA-based hydrogel scaffolds, contributing to the advancement of tissue engineering. Unlike other GAGs, HA is a free glycan and does not possess a sulfation modification. Highly sulfated HA, in which the hydroxyl groups of GlcA and GlcNAc on the HA chain are chemically sulfated, has been developed in recent years, and its application to pluripotent stem cells is underway. Generally, maintaining human pluripotent stem cells in an undifferentiated state requires activation of Erk1/1 signaling by the addition of FGF2. Surprisingly, it has been reported that the undifferentiated state of human iPS cells can be maintained by adding highly sulfated HA under FGF2-free conditions (6) (Figure 3). Furthermore, highly sulfated HA has been found to have stronger FGF2-binding ability than heparin (i.e., high content of IdoA and highly sulfated modification in HS, which is known to bind strongly to FGF2). Collectively, these findings indicate the possibility of utility of HA in regenerative medicine and cell therapy, as well as the application of hyper-sulfated HA to stem cell research.
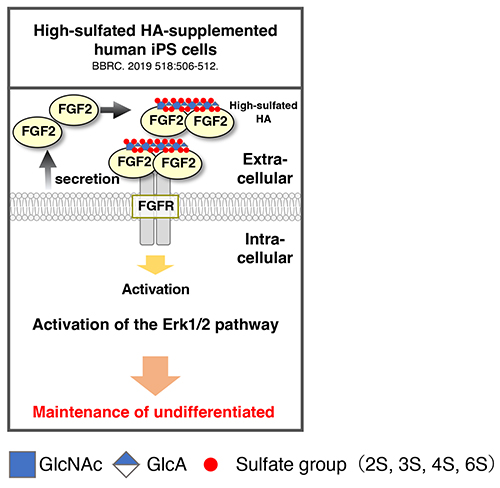
Figure 3. Role of sulfated HA in pluripotent stem cells
Scheme shows the reported function of sulfated HA. High-sulfated HA exhibits strong binding to FGF2 and promotes signaling transduction by autocrine FGF2.
HA, hyaluronan; FGF, fibroblast growth factor 2; FGFR, fibroblast growth factor receptor.
|
Kasumi Hirano1, Shoko Nishihara2
(1 Biomedical Research Institute, National Institute of Advanced Industrial Science and Technology (AIST)
2 Glycan & Life System Integration Center (GaLSIC), Soka University)
| References |
| (1) |
Nishihara S: Glycans in stem cell regulation: from Drosophila tissue stem cells to mammalian pluripotent stem cells. FEBS Lett. 592, 3773-3790, 2018 |
| (2) |
Izumikawa T, Sato B, Kitagawa H: Chondroitin sulfate is indispensable for pluripotency and differentiation of mouse embryonic stem cells. Sci. Rep. 4, 3701, 2014 |
| (3) |
Ogura C, Nishihara S: Dermatan-4-O-Sulfotransferase-1 Contributes to the Undifferentiated State of Mouse Embryonic Stem Cells. Front. Cell. Dev. Biol. 9, 733964, 2021 |
| (4) |
Ogura C, Hirano K, Mizumoto S, Yamada S, Nishihara S: Dermatan sulphate promotes neuronal differentiation in mouse and human stem cells. J. Biochem. 169, 55-64, 2021 |
| (5) |
Nagai Y, Nakao H, Kojima A, Komatsubara Y, Ohta Y, Kawasaki N, Kawasaki N, Toyoda H, Kawasaki T: Glycan Epitopes on 201B7 Human-Induced Pluripotent Stem Cells Using R-10G and R-17F Marker Antibodies. Biomolecules 11, 508, 2021 |
| (6) |
Miura T, Yuasa N, Ota H, Habu M, Kawano M, Nakayama F, Nishihara S: Highly sulfated hyaluronic acid maintains human induced pluripotent stem cells under feeder-free and bFGF-free conditions. Biochem. Biophys. Res. Commun. 518, 506-512, 2019 |
|
|---|









