 |
The nematode C. elegans is a widely used model organism. The glycosaminoglycans (GAGs) found in C. elegans are chondroitin (Chn) and heparan sulfate (HS). No hyaluronan has been found in C. elegans (1). Here we provide a brief overview of both Chn proteoglycans and HS proteoglycans of the nematode.
Chondroitin (Chn)
Most of the chondroitin (Chn) in C. elegans is not sulfated, with the amount of sulfated Chn (sulfation at the hydroxy group of 4-O position on N-acetyl-galactosamine residue) comparable to that of HS. While Chn sulfation has been implicated in oxidative stress resistance, its other functions remain unclear. It may potentially be involved in neural network formation and nerve regeneration, among other possibilities, which would make it an area for future research (2).
There are two genes involved in the synthesis of chondroitin: sqv-5 and mig-22. The sqv-5 gene encodes chondroitin synthase, and the mig-22 gene encodes chondroitin polymerizing factor. Inhibition of either gene through RNAi or genetic knockout results in inhibition of early embryonic cell division (both cytoplasmic and nuclear) (3). As shown in Figure 1, simultaneous inhibition of the genes cpg-1 and cpg-2, which code for the protein portion (core protein) of Chn proteoglycan (CPG), results in abnormal cell division of early embryos. The cell membrane is stained with a green fluorescent dye (lower image), and the upper image is a differential interference contrast microscopy image. Inhibition of the synthesis of both core proteins results in the destabilization of cleavage furrows of the early cleavage stage embryo (Figure 1a), and the cleavage furrows gradually regress over time (Figure 1b, c, d), ultimately resulting in the disappearance of all partitions of the early embryos, forming a single, multinucleated giant cell (Figure 1e). The requirement for Chn synthesis in early embryonic development has also been demonstrated in mice, indicating that Chn is an essential molecule in early embryogenesis in mammals as well. Additionally, Chn synthesis is indispensable for the formation of the vulva, the smallest organ in the world, in C. elegans (1).
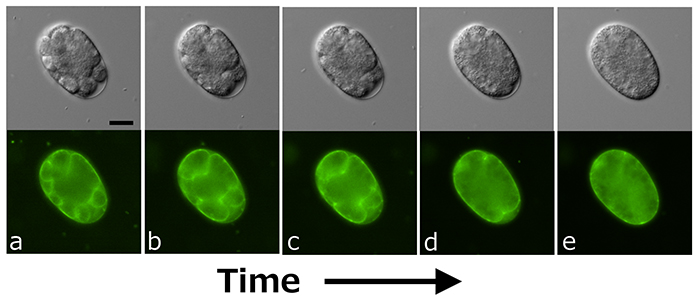
Figure 1. Aberrant cell division of an early embryo upon simultaneous inhibition of cpg-1 and cpg-2 genes (see the main text for details).
Scale bar: 20 μm.
|
Currently, 24 core protein genes for CPG (cpg-1 to cpg-24) have been identified in the nematode, several of which have domains in common with mammalian genes (4). However, only three genes, cpg-1, cpg-2, and cpg-14, have been found to be involved in early embryonic cell division. The predicted three-dimensional (3D) structures of all CPG proteins, using AlphaFold2, are shown in Figure 2. Almost all core proteins have intrinsically disordered regions (IDRs) (shown in green in the figure) that do not adopt a specific structure, and Chn is often attached to this region. In C. elegans oocytes, CPG-1 and CPG-2 are present in membrane granules called cortical granules, which contain caveolin. CPG-1 and CPG-2 are transported to the cleavage furrow during oocyte-to-embryo transition followed by early embryonic cell division. The 3D structures of the two proteins with their IDRs (Figure 3) are similar to those of the protein LAF-1, which forms liquid droplets (P-granules) by liquid-liquid phase separation (Figure 3). As shown in Figure 3, human syndecan-4 (SYND4) is a proteoglycan (PG) with IDRs, and has a similar 3D structure to many C. elegans CPGs including CPG-9 and CPG-19 (Figures 2 and 3). SYND4 is known to be modified by both HS and Chn. The molecule is essential for cytoplasmic division of cultured human cells and is localized in the midbody of dividing cells (5). These observations suggest that CPG core proteins with IDRs may interact with other molecules as a part of liquid droplets, raising the possibility that the signal for cell division from the nucleus, which is rich with liquid droplets, may be transmitted to the cell surface via liquid droplets containing CPG core proteins and cortical granules. This is an important issue for further research.
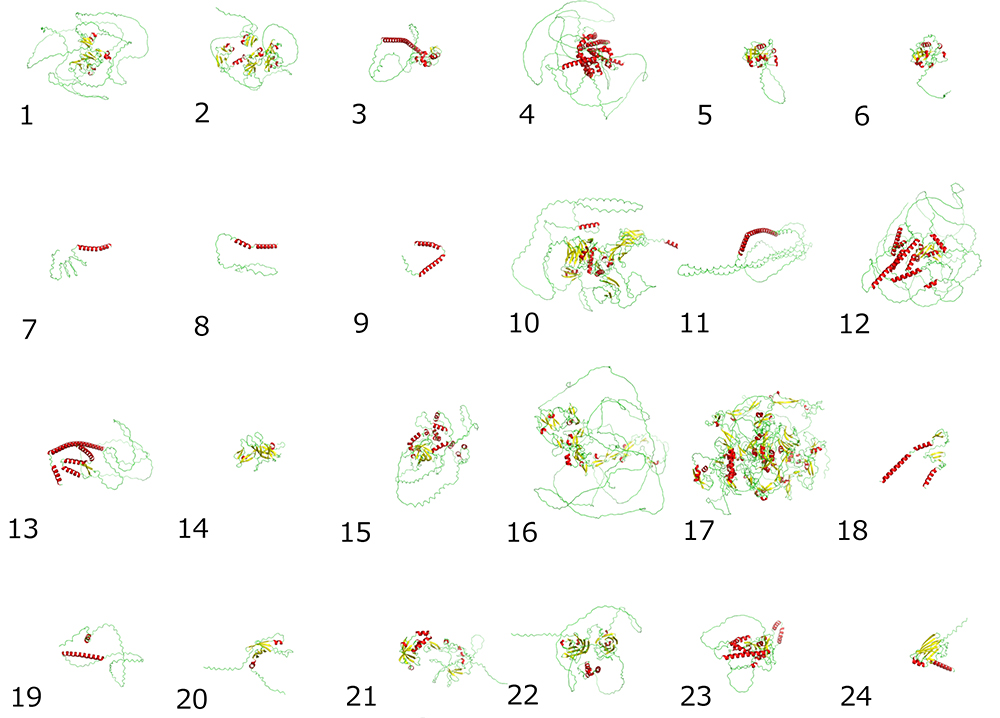
Figure 2. Predicted 3D structures of 24 CPG proteins from the AlphaFold Protein Structure Database.
(Numbers indicate cpg gene numbers)
|
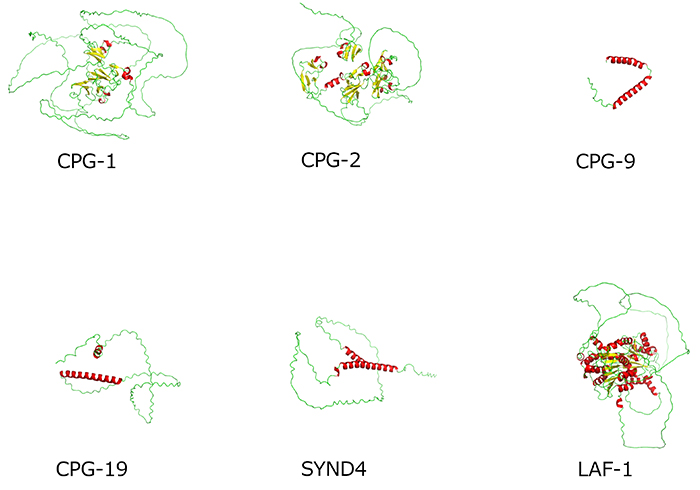
Figure 3. Comparison of predicted 3D structures of CPG proteins with IDRs from the AlphaFold Protein Structure Database
Note that LAF-1 is not a CPG and included as a reference.
|
Heparan sulfate (HS)
In C. elegans, HS accounts for approximately 0.2% of the amount of Chn and is synthesized by the products of rib-1 and rib-2 genes, which are orthologs of the human EXT1 and EXTL3 genes, respectively. These two proteins form a complex and work together, so inhibiting the function of either rib-1 or rib-2 gene disturbs HS synthesis. When the function of either rib-1 or rib-2 is inhibited, it can lead to larval lethality due to abnormalities in the ventral enclosure morphogenesis after the gastrulation stage. If the inhibition is weak, abnormalities in the formation of the neural circuitry and pharynx are observed. These abnormalities are similar to the effects of inhibiting the synthesis of core protein of HSPGs at each stage, and to the effects of inhibiting the sulfation of proteins in general. Unlike the abnormalities observed in cell division during early embryogenesis when Chn synthesis is inhibited, inhibition of HS synthesis does not result in these abnormalities (1). Known core proteins of HSPGs include syndecan (encoded by the sdn-1), glypicans (lon-2 and gpn-1), perlecan (unc-52), agrin (agr-1), and type XVIII collagen (cle-1), which also encodes CPG-10.
Kazuya Nomura
(Department of Medical Biochemistry, Kurume University School of Medicine)
| References |
| (1) |
Nomura K, Akiyoshi S, Matsuda A, Nomura KH: Glycosaminoglycans and glycosylphosphatidylinositol-anchor proteins in development of Caenorhabditis elegans. In Glycoscience: Biology and Medicine (Taniguchi N, Endo T, Hart GW, Seeberger PH, Wong, C-H eds), 817–824, Springer Japan, Tokyo, 2015 |
| (2) |
Izumikawa T, Dejima K, Watamoto Y, Nomura KH, Kanaki N, Rikitake M, Tou M, Murata D, Yanagita E, Kano A, Mitani S, Nomura K, Kitagawa H: Chondroitin 4-O-sulfotransferase is indispensable for sulfation of chondroitin and plays an important role in maintaining normal life span and oxidative stress responses in nematodes. J. Biol. Chem. 291, 23294–23304, 2016 |
| (3) |
Mizuguchi S, Uyama T, Kitagawa H, Nomura KH, Dejima K, Gengyo-Ando K, Mitani S, Sugahara K, Nomura K: Chondroitin proteoglycans are involved in cell division of Caenorhabditis elegans. Nature 423, 443–448, 2003 |
| (4) |
Noborn F, Larson G: Characterization of C. elegans chondroitin proteoglycans and their large functional and structural heterogeneity; evolutionary aspects on structural differences between humans and the nematode. Adv. Exp. Med. Biol. 21, 155–170, 2021 |
| (5) |
Addi C, Presle A, Frémont S, Cuvelier F, Rocancourt M, Milin F, Schmutz S, Chamot-Rooke J, Douché T, Duchateau M, Giai Gianetto Q, Salles A, Ménager H, Matondo M, Zimmermann P, Gupta-Rossi N, Echard A: The Flemmingsome reveals an ESCRT-to-membrane coupling via ALIX/syntenin/syndecan-4 required for completion of cytokinesis. Nat. Commun. 11, 1941, 2020 |
Jun. 15, 2023
|
|---|









