 |
Knockout (KO) mice deficient in hyaluronan synthase (HAS) genes are powerful tools for analyzing hyaluronan (HA) function in vivo, both as laboratory animals and as disease models. Of the three HAS genes, mice deficient in Has1 and Has3 genes are viable and fertile, allowing functional analysis in adults. In contrast, Has2 KO mice are embryonically lethal at embryonic day 9.5, mainly due to the failure of cardiac morphogenesis, yolk sac dysplasia, and abnormal blood vessel formation (1). During cardiac morphogenesis, the endocardial cushion is formed in the atrioventricular canal that connects the atria and ventricles. In Has2 KO embryos, the endocardial-to-mesenchymal transformation is impaired during the formation of endocardial cushions (Fig. 1). Conditional knockout (cKO) mice, which allow for temporal and tissue-specific gene deletions, are particularly useful for analyzing the function of Has2 in a tissue-specific manner at each embryonic stage or in adults.
Has1 KO and Has1/Has3 double KO (dKO) mice showed no differences in Achilles tendon morphology, but impaired post-natal formation of retrocalcaneal bursa filled with HA-rich fluid (2), suggesting an important role of Has1-mediated HA metabolism in the formation and maintenance of retrocalcaneal bursa.
Cutaneous wound closure was accelerated in a wound healing model of Has1/Has3 dKO mice (3). Consistent with abnormal wound healing, dKO mice showed a marked decrease in epidermal and dermal HA levels, increased neutrophil efflux from cutaneous blood vessels, and enhanced myofibroblast differentiation. Myofibroblast differentiation, which is regulated by the TGF-β signaling pathway, is an important process in skin wound healing. In primary dermal fibroblasts isolated from Has1/Has3 dKO mice, a non-canonical TGF-β signaling pathway through p38 MAP kinase activation promoted myofibroblast differentiation.
HA is a major component of the brain's extracellular matrix. Has1 KO, Has2 cKO, and Has3 KO mice all exhibited epileptiform activity (4). Of these, Has3 KO mice had the highest frequency of epileptic seizures and showed a marked decrease in hippocampal HA matrix. Due to the remarkable hydration capacity of HA, a deficiency of HA caused a decrease in the extracellular space volume in the brain. Reduced extracellular space volume promotes epileptiform activity primarily by enhancing ephaptic interactions and increasing extracellular potassium concentrations.
Apolipoprotein E (ApoE) KO mice showed increased Has3 expression in macrophages that accumulate in atherosclerotic lesions. Macrophage accumulation and inflammation in atherosclerotic lesions were decreased in Has3/ApoE dKO mice, resulting in reduced atherosclerosis development (5). Furthermore, HA accumulation in the arterial tunica media was reduced and neointimal hyperplasia was strongly suppressed in a carotid artery ligation model of Has3 KO mice (5). The expression of genes related to vascular smooth muscle cell activation and migration was downregulated in Has3 KO mice, suggesting the involvement of Has3-synthesized HA in neointimal hyperplasia. In a mouse model of myocardial infarction caused by ischemia and reperfusion, deletion of the Has3 gene impaired the contractile function of the myocardium, resulting in symptoms of heart failure (6). CD4-positive T cell subsets are believed to play an important role in the healing process of myocardial infarction. Both Th1 and Treg cells, CD4-positive T cell subsets, were reduced in the hearts of Has3 KO mice after myocardial infarction, suggesting that HA synthesized by Has3 is an important determinant of physiological T cell responses in cardiac healing after myocardial infarction.
Tissue-specific deletion of the Has2 gene in the developing limb resulted in abnormalities in limb skeletal growth, finger patterning, chondrocyte maturation, and joint formation (7). In the limbs of Has2 mutant mice, the skeletal elements were severely shortened and the proximal phalanges were duplicated. Furthermore, skeletal growth plates were severely disrupted and the number of hypertrophic chondrocytes was significantly reduced in the mutant mice. These results suggest that HA synthesized by Has2 plays an important role in skeletal growth and maturation as a major component of the extracellular matrix.
As described above, analyses of Has KO mice revealed the physiological functions of HA in embryonic development, morphogenesis, and wound healing, and the significance of HA metabolism in diseases such as epilepsy and atherosclerosis. A detailed analysis of Has KO mice will provide a more complete understanding of the diverse functions of HA.
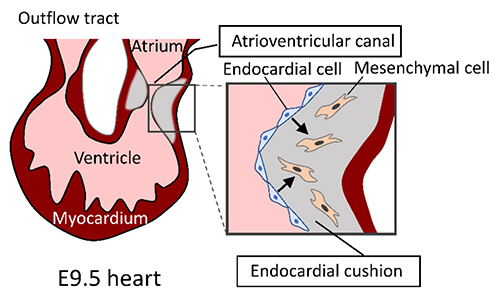
Fig. 1. Endocardial-to-mesenchymal transformation during cardiac morphogenesis.
During cardiac morphogenesis, endocardial cells of the atrioventricular canal transform into mesenchymal cells that invade and remodel the endocardial cushion. HA-rich extracellular matrix plays an important role in the cell migration and transformation involved in the formation of the endocardial cushion.
|
Naoki Itano
(Faculty of Life Sciences, Kyoto Sangyo University)
| References |
| (1) |
Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A Jr, Kubalak S, Klewer SE, McDonald JA: Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J. Clin. Invest. 106, 349-360, 2000 |
| (2) |
Sikes KJ, Renner K, Li J, Grande-Allen KJ, Connell JP, Cali V, Midura RJ, Sandy JD, Plaas A, Wang VM: Knockout of hyaluronan synthase 1, but not 3, impairs formation of the retrocalcaneal bursa. J. Orthop. Res. 36, 2622-2632, 2018 |
| (3) |
Mack JA, Feldman RJ, Itano N, Kimata K, Lauer M, Hascall VC, Maytin EV: Enhanced inflammation and accelerated wound closure following tetraphorbol ester application or full-thickness wounding in mice lacking hyaluronan synthases Has1 and Has3. J. Invest. Dermatol. 132, 198-207, 2012 |
| (4) |
Arranz AM, Perkins KL, Irie F, Lewis DP, Hrabe J, Xiao F, Itano N, Kimata K, Hrabetova S, Yamaguchi Y: Hyaluronan deficiency due to Has3 knock-out causes altered neuronal activity and seizures via reduction in brain extracellular space. J. Neurosci. 34, 6164-6176, 2014 |
| (5) |
Fischer JW: Role of hyaluronan in atherosclerosis: Current knowledge and open questions. Matrix Biol. 78-79, 324-336, 2019 |
| (6) |
Piroth M, Gorski DJ, Hundhausen C, Petz A, Gorressen S, Semmler D, Zabri H, Hartwig S, Lehr S, Kelm M, Jung C, Fischer JW: Hyaluronan synthase 3 is protective after cardiac ischemia-reperfusion by preserving the T cell response. Matrix Biol. 112, 116-131, 2022 |
| (7) |
Matsumoto K, Li Y, Jakuba C, Sugiyama Y, Sayo T, Okuno M, Dealy CN, Toole BP, Takeda J, Yamaguchi Y, Kosher RA: Conditional inactivation of Has2 reveals a crucial role for hyaluronan in skeletal growth, patterning, chondrocyte maturation and joint formation in the developing limb. Development 136, 2825-2835, 2009 |
Jun. 15, 2023
|
|---|







