 |
Dermatan sulfate (DS) is a linear glycosylated chain of proteoglycans which is expressed at cell surface and extracellular matrix. DS is distributed in various tissues such as skin, tendon, and aorta. DS and chondroitin sulfate (CS) are covalently attached on serine residues of specific core proteins of proteoglycans via common tetrasaccharide linker region, glucuronic acid-β1-3galactose-β1-3galactose-β1-4xyloseβ1-O-. Chondroitin contains disaccharide units consisting of glucuronic acid (GlcA) snd N-acetylgalactosamine (GalNAc). Dermatan sulfate epimerase (DSE) converts chondroitin into dermatan by catalyzing the conversion of GlcA to iduronic acid (IdoA). Dermatan 4-O-sulfotransferase-1 (D4ST1), encoded by Chst14 gene, catalyzes the transfer of a sulfate group to GalNAc residue of dermatan leading to mature DS. DSE catalyzes epimerization of chondroitin and dermatan in either direction. D4ST1 deficiency suppresses conversion of chondroitin into dermatan and then dermatan is converted into chondroitin (Figure 1).
Gene-deleted mice of Chst14 (Chst14 KO mice) showed loss of DS in some tissues (1,2). On the other hand, Dse or Dse-like (Dsel) gene-deleted mice showed significant decrease in IdoA residue in DS chain but not complete dissipation (3,4). Double knockout mice of Dse and Dselgenes showed complete loss of IdoA residue in DS chain (5). However, perinatal lethality made detailed analysis of the phenotypes of double knockout mice difficult (5). This article organizes the published data of Chst14 KO mouse as a DS deficient mouse.
Many Chst14 KO embryos showed embryonic lethality with ischemic and/or necrotic phenotype of placenta (6). Thinning of basement membranes of capillary of placental villus were observed in the KO embryos (6). Birth rates were affected by genetic background of the mice. Though all embryos were died at prenatal period with C57BL/6 genetic background (7), backcrossing to BALB/c genetic background improved the birth rates, which was 8% of the whole offspring of heterozygous breeding pairs (8). After the perinatal period, lifespan of viable Chst14 KO mice was similar to that of wild type (2,7). Body weights were decreased in Chst14 KO mice from pups to adults (2,7). In Chst14 KO mice, skin fragility with loosened collagen bundles were observed by analyses using H&E staining as well as transmission electron microscopy (TEM) (1,7). It is known that IdoA in DS is conformationally more flexible than GlcA in CS. The bound angle between GlcA and adjacent GalNAc in CS is relatively fixed, whereas that between IdoA and GalNAc in DS is rather adjustable (9). In electron microscopy with cupromeronic blue staining, which can visualize sulfated glycosaminoglycans, disorganized collagen fibrils with deformation of glycosaminoglycan chains (these were altered into rod-shaped from round-shaped) which were attached at one end to collagen fibrils were found in the skin of Chst14 KO mice (1). These reports suggested that deformation of collagen fibrils in extracellular matrix was the cause of decreased strength of skin and deformation of these tissues. In addition, loss of DS induced kinked tail and thoracickyphosis (2,7). Furthermore, Chst14 KO mice showed myopathy-related phenotypes, decreased motor functions such as grip strength, voluntary activity, and running speed (2). Gene deletion of Chst14 accelerated nerve regeneration and functional recovery after femoral nerve injury in the mice (7). Though brain weights or gross anatomy of Chst14 KO mice were not significantly different compared with wild-type, deficit in spatial learning and memory was observed (10).
These reports suggested that DS was essential for connective tissue morphology and strength via support of collagen fibrils oriented uniformly to form well-organized fibers in the mice. In addition, it was also suggested that DS was related with the functions of skeletal muscle and cranial nervous system. More detailed analysis of the in vivo functions of DS is expected in the future. Furthermore, because mutations in CHST14 genes are the cause of musculocontractural Ehlers-Danlos syndrome in human (11) (see “A genetic disorder caused by defective enzymes involving dermatan sulfate biosynthesis: musculocontractural Ehlers-Danlos syndrome”), Chst14 KO mouse is a promising model animal of the disease.
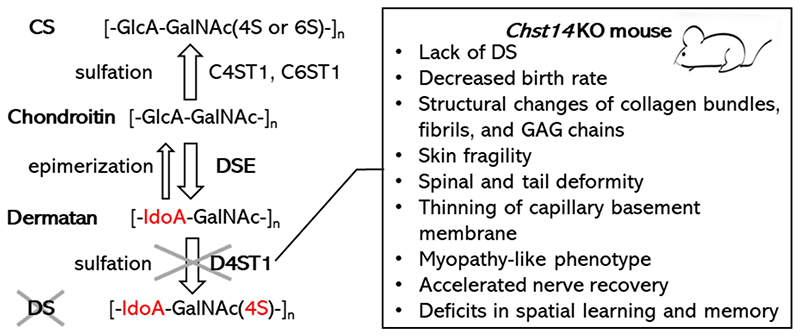
Figure 1. Enzymes related to CS and DS synthesis and phenotypes of Chst14 KO mouse
GalNAc residues of CS are commonly sulfated at the hydroxy group of C-4 and/or C-6 position by chondroitin 4-O-sulfotransferase 1 (C4ST1) and/or chondroitin 6-O-sulfotransferase 1 (C6ST1), respectively. GlcA residues of chondroitin are epimerized into IdoA residues by DSE and thereby chondroitin is converted into dermatan. GalNAc residues of DS are commonly sulfated at the hydroxy group of C4 position by D4ST1. Gene deletion of Chst14 induces D4ST1 deficiency and phenotypes of the KO mice.
|
Takahiro Yoshizawa
(Research Center for Advanced Science and Technology, Shinshu University)
| References |
| (1) |
Hirose T, Mizumoto S, Hashimoto A, Takahashi Y, Yoshizawa T, Nitahara-Kasahara Y, Takahashi N, Nakayama J, Takehana K, Okada T, Nomura Y, Yamada S, Kosho T, Watanabe T: Systematic investigation of the skin in Chst14-/- mice: A model for skin fragility in musculocontractural Ehlers-Danlos syndrome caused by CHST14 variants (mcEDS-CHST14). Glycobiology 31, 137-150, 2021 |
| (2) |
Nitahara-Kasahara Y, Mizumoto S, Inoue Y U, Saka S, Posadas-Herrera G, Nakamura-Takahashi A, Takahashi Y, Hashimoto A, Konishi K, Miyata S, Masuda C, Matsumoto E, Maruoka Y, Yoshizawa T, Tanase T, Inoue T, Yamada S, Nomura Y, Takeda S, Watanabe A, Kosho T, Okada T: A new mouse model of Ehlers-Danlos syndrome generated using CRISPR/Cas9-mediated genomic editing. Dis. Model. Mech. 14, dmm048963, 2021 |
| (3) |
Maccarana M, Kalamajski S, Kongsgaard M, Magnusson S P, Oldberg A, Malmstrom A: Dermatan sulfate epimerase 1-deficient mice have reduced content and changed distribution of iduronic acids in dermatan sulfate and an altered collagen structure in skin. Mol. Cell. Biol. 29, 5517-5528, 2009 |
| (4) |
Bartolini B, Thelin M A, Rauch U, Feinstein R, Oldberg A, Malmstrom A, Maccarana M: Mouse development is not obviously affected by the absence of dermatan sulfate epimerase 2 in spite of a modified brain dermatan sulfate composition. Glycobiology 22, 1007-1016, 2012 |
| (5) |
Stachtea X N, Tykesson E, van Kuppevelt T H, Feinstein R, Malmstrom A, Reijmers R M, Maccarana M: Dermatan sulfate-free mice display embryological defects and are neonatal lethal despite normal lymphoid and non-lymphoid organogenesis. PLoS One 10, e0140279, 2015 |
| (6) |
Yoshizawa T, Mizumoto S, Takahashi Y, Shimada S, Sugahara K, Nakayama J, Takeda S, Nomura Y, Nitahara-Kasahara Y, Okada T, Matsumoto K, Yamada S, Kosho T: Vascular abnormalities in the placenta of Chst14-/- fetuses: implications in the pathophysiology of perinatal lethality of the murine model and vascular lesions in human CHST14/D4ST1 deficiency. Glycobiology 28, 80-89, 2018 |
| (7) |
Akyuz N, Rost S, Mehanna A, Bian S, Loers G, Oezen I, Mishra B, Hoffmann K, Guseva D, Laczynska E, Irintchev A, Jakovcevski I, Schachner M: Dermatan 4-O-sulfotransferase1 ablation accelerates peripheral nerve regeneration. Exp. Neurol. 247, 517-530, 2013 |
| (8) |
Shimada S, Yoshizawa T, Takahashi Y, Nitahara-Kasahara Y, Okada T, Nomura Y, Yamanaka H, Kosho T, Matsumoto K: Backcrossing to an appropriate genetic background improves the birth rate of carbohydrate sulfotransferase 14 gene-deleted mice. Exp. Anim. 69, 407-413, 2020 |
| (9) |
Miyake N, Kosho T, Mizumoto S, Furuichi T, Hatamochi A, Nagashima Y, Arai E, Takahashi K, Kawamura R, Wakui K, Takahashi J, Kato H, Yasui H, Ishida T, Ohashi H, Nishimura G, Shiina M, Saitsu H, Tsurusaki Y, Doi H, Fukushima Y, Ikegawa S, Yamada S, Sugahara K, Matsumoto N: Loss-of-function mutations of CHST14 in a new type of Ehlers-Danlos syndrome. Hum. Mutat. 31, 966-974, 2010 |
| (10) |
Li Q, Wu X, Na X, Ge B, Wu Q, Guo X, Ntim M, Zhang Y, Sun Y, Yang J, Xiao Z, Zhao J, Li S: Impaired cognitive function and altered hippocampal synaptic plasticity in mice lacking dermatan sulfotransferase Chst14/D4st1. Front. Mol. Neurosci. 12, 26, 2019 |
| (11) |
Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, Bloom L, Bowen J M, Brady A F, Burrows N P, Castori M, Cohen H, Colombi M, Demirdas S, De Backer J, De Paepe A, Fournel-Gigleux S, Frank M, Ghali N, Giunta C, Grahame R, Hakim A, Jeunemaitre X, Johnson D, Juul-Kristensen B, Kapferer-Seebacher I, Kazkaz H, Kosho T, Lavallee M E, Levy H, Mendoza-Londono R, Pepin M, Pope F M, Reinstein E, Robert L, Rohrbach M, Sanders L, Sobey G J, Van Damme T, Vandersteen A, van Mourik C, Voermans N, Wheeldon N, Zschocke J, Tinkle B: The 2017 international classification of the Ehlers-Danlos syndromes. Am. J. Med. Genet. C Semin. Med. Genet. 175, 8-26, 2017 |
|
|---|







