|
Involvement of Protein Tyrosine Phosphatase Receptor Type Z (Ptprz/PTP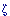 /RPTP /RPTP ) Signaling in Gastric Ulcer Induction by VacA of Helicobacter Pylori ) Signaling in Gastric Ulcer Induction by VacA of Helicobacter Pylori
|
|
|
 |
Protein tyrosine phosphatase receptor type Z (Ptprz/PTP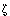 /RPTP /RPTP ) is a receptor-like protein-tyrosine phosphatase which is mainly expressed in the central nervous system as chondroitin sulfate (CS) proteoglycans (see PG-B05). Recently, it was revealed that Ptprz is a receptor for the vacuolating cytotoxin VacA produced by Helicobacter pylori (H. pylori), which is a primary pathogenic factor for the gastric ulcer (1). ) is a receptor-like protein-tyrosine phosphatase which is mainly expressed in the central nervous system as chondroitin sulfate (CS) proteoglycans (see PG-B05). Recently, it was revealed that Ptprz is a receptor for the vacuolating cytotoxin VacA produced by Helicobacter pylori (H. pylori), which is a primary pathogenic factor for the gastric ulcer (1).
Analyses on mouse and human tissues have shown the expression of Ptprz in the stomach, although the expression level is lower by one order of magnitude than that in the brain (1). Among the three splicing isoforms, Ptprz-B was the main species in the stomach. Of note is that Ptprz-B was not modified with CS in the stomach.
It is known that VacA binds to specific cell receptors and is internalized by cells. After internalization, VacA localizes in the endocytic-endosomal compartment, from which vacuoles originate by the anion channel activity of VacA. When mice were orally treated with VacA, wild-type mice showed heavy bleeding in the stomach and developed gastric ulcer. Surprisingly, there was no epithelial damage in any of the Ptprz-/- mice treated equally (Figure 1, and ref. 1). Because there was no difference between the two genotypes in mucosal damage induced by administration of indomethacin or ethanol in hydrochloric acid (1), Ptprz appears to be specifically relevant to the pathogenesis of VacA-induced gastric ulcers. |
|
|
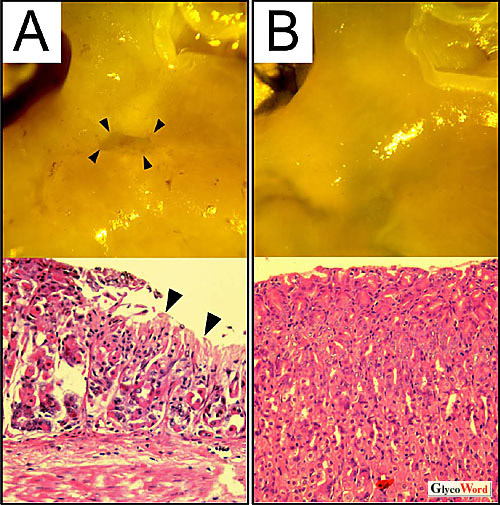 |
Fig. 1 Gastric tissues treated with VacA.
Gastric ulcers appeared in the stomach of wild-type mice (A) but not of Ptprz-/- mice (B).
Upper: Stereomicroscopic appearance of the inside of stomachs
Lower: Gastric sections stained with homatoxylin and eosin. |
|
|
|
As the vacuolating activity had been believed to be the major cytotoxic activity of VacA, incorporation of VacA into the gastric epithelial cells was expected to be decreased in Ptprz-/- mice. However, VacA was detected in the cytoplasm of a number of epithelial cells equally in both wild-type and Ptprz-/- mice. In addition, there was no difference in the vacuole development induced by VacA between wild-type and Ptprz-/- cells in the primary culture of gastric epithelial cells (1). These results suggest that VacA induces gastric ulcer through signal transduction of Ptprz but not through cellular incorporation and vacuolation.
When the primary culture of gastric epithelial cells was tested on a reconstituted basement membrane, it was found that only wild-type cells began to detach after VacA treatment (1). Git1 had been identified as a substrate of Ptprz (2). Git1 is a multidomain protein that is thought to function as an integrator of signaling pathways controlling cell adhesion and cytoskeletal organization. Then, it was speculated that VacA acts as a ligand for Ptprz, and causes cellular detachment in wild-type cells through Git1. In cells transfected with Ptprz, the tyrosine-phosphorylation level of Git1 was dose-dependently increased by VacA. In contrast, the tyrosine-phosphorylation level of Git1 was not increased in cells transfected with an inactive Ptprz mutant. Taken all together, binding of VacA to Ptprz leads to inactivation of PTP, and then, the tyrosine phosphorylation of cellular proteins including Git1 is increased. This probably impairs the adhesion between the epithelial cell and extracellular matrix, and results in the ulcerogenesis in vivo (Figure 2). In support of this view, pleiotrophin, one of the endogenous ligands of Ptprz, also induced severe gastritis in wild-type mice but not in Ptprz-/- mice when administered orally (1).
It has been pointed out that H. pylori infection is also relevant to induction of gastric cancers. The finding that VacA induces aberrant tyrosine-phosphorylation signals in cells is very intriguing, because it could be a factor in carcinogenesis. |
|
|
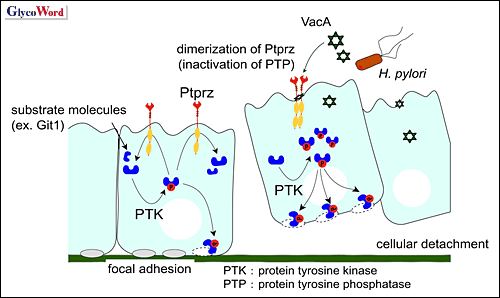 |
Fig. 2 Molecular mechanism of induction of gastric ulcer by VacA.
VacA binds to Ptprz as a ligand and leads to inactivation of PTP. This aberrant tyrosine-phosphorylation signal of cellular proteins including Git1 leads to detachment of gastric epithelial cells from the basement membrane. Finally, tissue damage begins by gastric acid and pepsin, resulting in the development of gastric ulcers. |
|
|
|
|
|
Masaharu Noda
(Division of Molecular Neurobiology, National Institute for Basic Biology) |
|
|
|
| References |
(1) |
Fujikawa A, Shirasaka D, Yamamoto S, Ota H, Yahiro K, Fukada M, Shintani T, Wada A, Aoyama N, Hirayama T, Fukamachi H, Noda M: Mice deficient in protein tyrosine phosphatase receptor type Z are resistant to gastric ulcer induction by VacA of Helicobacter pylori. Nat. Genet. 33, 375-381, 2003 |
|
(2) |
Kawachi H, Fujikawa A, Maeda N, Noda M: Identification of GIT1/Cat-1 as a substrate molecule of protein tyrosine phosphatase 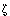 / /  by the yeast substrate-trapping system. Proc. Natl. Acad. Sci. USA. 98, 6593-6598, 2001 by the yeast substrate-trapping system. Proc. Natl. Acad. Sci. USA. 98, 6593-6598, 2001 |
|
|
|
|
Links
|
|
PG-B05 Functional Roles of Protein Tyrosine Phosphatase zeta(PTP zeta/RPTPß) (Masaharu Noda) |
|
|
|
NS-A01 Functional Roles of Chondroitin Sulfate in Neural Network Formation
(Nobuaki Maeda) |
|
|
|
|
|
| Mar.31, 2004 |
|
|
|
|
|
|
|




