

 |
 |
Functional Role of the HNK-1 Carbohydrate in the Nervous System |
|||||||||||||||||||||||
 |
The HNK-1 carbohydrate is characteristically expressed on
a series of cell adhesion molecules, and the expression is spatially and
temporally regulated during the development of the nervous system, suggesting
that the HNK-1 carbohydrate plays an important role in the formation of
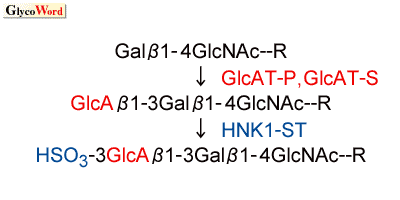
the neural network1). Glucuronyltransferase(s) and sulfotransferase(s) are key enzymes for this carbohydrate biosynthesis, because the structure of the HNK-1 carbohydrate is a sulfated trisaccharide, HSO3-3GlcAb |
||||||||||||||||||||||
 |
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
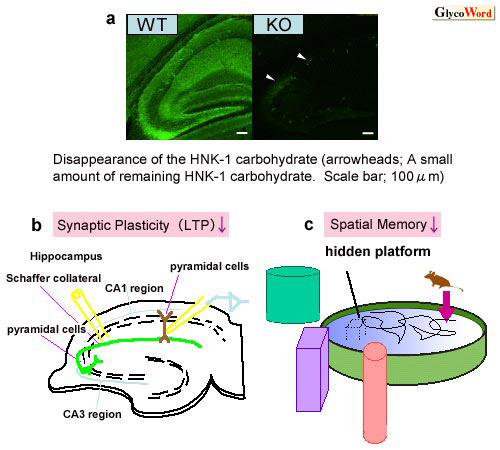
GlcAT-P was thought to be specific to the glycoprotein acceptors. However, the glucuronyltransferase activity of GlcAT-P gene-deficient mice toward the glycolipid acceptor disappeared almost completely as well as the activity toward the glycoprotein acceptor. Almost all the HNK-1 carbohydrate also disappeared in GlcAT-P gene-deficient mouse brain (Fig. 2-a), suggesting that GlcAT-P is the most predominant glucuronyltransferase responsible for the biosynthesis of the HNK-1 carbohydrate in brain.
|
|||||||||||||||||||||||
 |
|||||||||||||||||||||||
|
|||||||||||||||||||||||
|
The HNK-1 carbohydrate is highly expressed during the
period corresponding to synaptogenesis. We analyzed LTP in the CA1 region
to examine the effect of HNK-1 carbohydrate deficiency on synaptic plasticity.
High-frequency stimulation of afferent fibers gave rise to LTP of excitatory
synaptic transmission in wild type mice, while the magnitude of LTP
in the GlcAT-P gene-deficient mice was significantly lower than that
in wild-type mice (Fig. 2-b). In view of the reduced LTP, two types
of spatial learning tests were carried out. In the Morris water maze
test, the time taken to reach the hidden platform (escape latency) was
significantly longer for the GlcAT-P gene-deficient mice than wild-type
mice (Fig. 2-c). In the water-filled multiple T-maze task, the GlcAT-P
gene-deficient mice showed increased escape latencies to the goal arm
compared to wild-type mice. These results indicate that the HNK-1 carbohydrate
plays an important role in synaptic plasticity and spatial memory formation.
The HNK-1 ST gene-deficient mice was also generated and analyzed independently. Basal synaptic transmission in pyramidal cells in the CA1 region of the hippocampus was increased and LTP evoked by theta-burst stimulation was reduced in the HNK-1 ST gene-deficient mice. In the water maze test, HNK-1 ST gene-deficient mice showed impaired spatial learning. These lines of evidence clearly indicate that the HNK-1 carbohydrate plays an essential role in the higher functions of the brain. Recently, Jeffries et al. (2003) mapped both chromosome breakpoints of a balanced t(6;11)(q14.2;q25) chromosome translocation that segregates with a schizophrenia-like psychosis3). The chromosome 11 breakpoint is situated close to the telomere and the closest gene is GlcAT-P. As GlcAT-P-deficient mice show defects in hippocampal LTP and in spatial memory formation, the above authors propose that the translocation causes a positional effect on GlcAT-P, affecting expression levels. |
|||||||||||||||||||||||
| Shogo Oka (Graduate School of Pharmaceutical Sciences, Kyoto University) | |||||||||||||||||||||||
|
|||||||||||||||||||||||
| Jan. 24, 2005 | |||||||||||||||||||||||
|
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||