| Collectins and Their Roles in Host Defense |  |
|
 |
Collectin (collagen-like lectin) is a subgroup of C-type (i.e. Ca2+-dependent) animal lectins characterized by the presence of collagen-like sequences (Gly-Xaa-Yaa triplet). The collectins include three serum proteins, mannan-binding protein (MBP) (also called mannose-binding protein, mannan-binding lectin, MBL), bovine conglutinin and bovine collectin-43 (CL-43), and two lung surfactant proteins (SP-A and SP-D). The collectins bind to terminal non-reducing sugar residues, mannose, GlcNAc, fucose and glucose. SP-D binds to maltose. They play important roles in innate immunity without involvement of antibodies (1).
The collectins are characterized by their unusual primary structures and also by their unique three-dimensional structures. Each polypeptide (30kDa-45kDa, 222-355 amino acid residues) contains a short N-terminal cysteine-rich region followed by a collagen-like sequence, a short linker or "neck" region, and then by a globular C-terminal carbohydrate recognition domain (CRD). Three identical or very similar polypeptides (human SP-A) bind together by disulfide bonds in the N-terminal segments, a triple helix via the collagen-like regions and a triple coiled-coil of alpha-helices in the neck region to form a structural subunit. The collectins consist of 3-6 identical structural units, which are held together by further disulfide linkages at the N-terminus to organize "flower bouquet" structures (MBP, SP-A), or form tetramers, which have "X-like" (conglutinin, SP-D) structures (see Figure). Multimeric binding of CRDs within each collectin to clustered oligosaccharide on a surface provides high affinity.
MBP, conglutinin, and CL-43 are synthesized in liver and secreted into the circulation. SP-A and SP-D are mainly synthesized in the lung type II alveolar cells and Clara cells, and secreted into the alveolar space. The serum MBP content increases significantly after birth, and their levels vary greatly even among healthy adult individuals. The serum level increases after infection and remains elevated for longer periods compared with other typical acute phase reactants.
The collectins have a consistent feature in the gene organization that the signal peptide, N-terminal segment, and the first few Gly-Xaa-Yaa triplets are encoded by a single exon. The rest of the collagen-like sequences are encoded by one (MBL and SP-A) to four (SP-D and conglutinin) exons. The "neck" region and CRD are each encoded by a single exon (2). All the known human collectin genes have been found clustered on chromosome 10q22-23.
MBP can activate the complement system through the classical pathway (3). Upon binding to carbohydrate structures on the surface of microorganism, the lectin activates the Clr2Cls2 protease complex, like C1q (the overall structure of MBP is very similar to that of C1q), or a novel C1s-like protease, MBP-associated serine proteases (MASPs). These proteases, in turn, activate C2 and C4 of the classical complement pathway and bring about the killing and clearance of the targets. This MBP-mediated complement activation, called the lectin pathway, provides an additional mechanism of microorganism recognition by the complement system in the absence of specific antibodies. Three point mutations in the first exon of the human MBP gene result in MBP deficiency, which is associated with frequent infections in childhood and possibly also in adult. | |
|
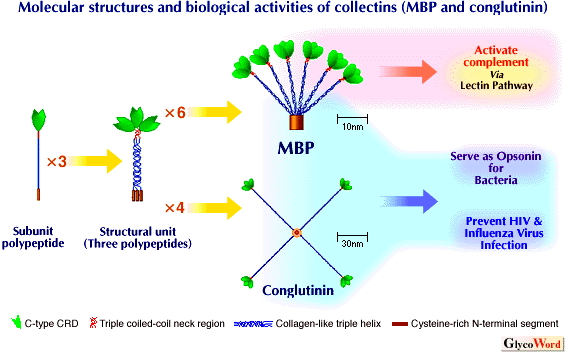
Figure Legend
Molecular structures and biological activities of the collectins (MBP and conglutinin).
MBP occurs not only as hexamers shown in figure, but also as trimers, tetramers and pentamers of the structural unit. SP-A consists of 6 structural units which is assembled into "flower bouquet" like MBP. Conglutinin and SP-D, consisting of the tetrameric structural units, are assembled into "X-like" shape. CL-43 molecules exists as monomers. The brown-colored regions seen in the centers of the MBP and the conglutinin molecules indicate the "hub" like structures. |
|
|
|
The collectins are known to act as opsonin in various circumstances. They recognize and bind non-host carbohydrates on microorganisms and particles and participate in the processing or elimination of such material by interaction with phagocytic cell receptors, C1q receptors (56kDa, 126kDa) through their collagen-like regions. An SP-A receptor of 210kDa is expressed on monocyte/alveolar macrophage. SP-A gene knockout mice have been shown to be susceptible to group B streptococcal infection, suggesting that lung surfactant collectins play a role in defense against pulmonary infection.
The collectins play an important role in the innate immune defense system. They bind to non-host carbohydrate structures present on the surface of a range of microorganisms, including bacteria, yeasts, fungi, parasitic protozoa and viruses, and exhibit antibacterial activity through killing mediated by the complement system or by promoting phagocytosis. | |
|
| Nobuko Kawasaki (Kyoto University, College of Medical Technology) | |
|
|
| References | (1) | Lu-J: Collectins: collectors of microorganisms for the innate immune system. BioEssays, 19, 509-518, 1997 |
| (2) | Kawasaki, N, Itoh, N, Kawasaki, T: Gene organization and 5'-flanking region sequence of conglutinin: a C-type mammalian lectin containing a collagen-like domain. Biochem. Biophys. Res. Commun. 198, 597-604, 1994 |
| (3) | Ikeda, K, Sannoh, T, Kawasaki, T, Kawasaki, N, Yamashina, I: Serum lectin with known structure activates complement through the classical pathway. J. Biol. Chem. 262, 7451-7454, 1987 |
| | |
| |
| Mar.15, 1998 |
|
| |
|
|
|
|



