

 |
 |
Heterogeneity of C-type lectins |  | |||||||||||||
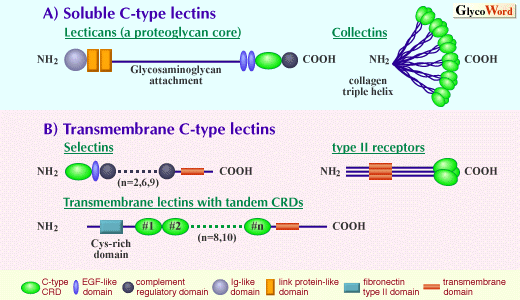
 | C-type lectins are calcium-dependent animal lectins that are carbohydrate-binding proteins of animal origin. Carbohydrate-binding activity of C-type lectins is based on the function of the carbohydrate recognition domain (CRD) whose structure is highly conserved among this family (1). Calcium is not only directly involved in the carbohydrate binding itself at the binding site (2) but contributes to the structural maintenance of the lectin domain that is essential for the lectin activity (3). The C-type CRDs are incorporated in a variety of contexts of molecular organization (Figure). This fact may reflect the importance of carbohydrate recognition in diverse biological functions. One can categorize two types of C-type lectins: soluble C-type lectins secreted from cells and transmembrane C-type lectins. The representative functions of the latter form are molecular recognition between cells, cell-cell adhesion and molecular uptake into cells. | |||||||||||||
 | ||||||||||||||
|
As a subfamily of soluble lectins, some proteoglycan core peptides (versican, aggrecan, neurocan and brevican) contain a C-type lectin domain, and the name 'lectican' has been proposed for this subfamily. These molecules contain a single C-type lectin domain in the vicinity of their carboxyl termini. Interestingly, the C-type lectin domain is located between two tandem epidermal growth factor (EGF)-like domains and a complement regulatory domain, which is the carboxyl terminal domain. The second type of soluble lectins is collectins. These molecules can participate in host defense mechanism, for example, through complement activation. This group includes serum mannose binding protein (MBP), lung surfactants SP-A and SP-D, and bovine serum protein conglutinin. These molecules consist of an amino-terminal collagen domain and a carboxyl terminal C-type lectin domain. Collagen-type triple helix produces a trimer of the lectin, and each trimer forms an oligomer. (Ref: Collectins and Their Roles in Host Defense) As a transmembrane C-type lectin, selectin is known as a family of cell-cell adhesion molecules. Selectins are involved in the adhesive interaction between leukocytes and vascular endothelial cells that are known to be an important step required for leukocyte extravasation. The selectin on the leukocyte side is L-selectin while those on the endothelial side are E- and P-selectins. (Ref: Cell Adhesion Molecules: Selectins) The second type of transmembrane C-type lectin is type II receptors. They have a single transmembrane domain with extracellular carboxyl termini and with cytoplasmic amino termini (type II transmembrane orientation). CRD is located at the extracellular carboxyl terminus. Typical examples of this group are asialoglycoprotein receptor of hepatocytes, macrophage galactose/N-acetylgalactosamine specific lectin, natural killer cell receptors, low affinity IgE receptor (CD23). They have a consensus amino acid motif responsible for the association with clathrin-coated vesicles within their cytoplasmic domains. Some of them (for example, asialoglycoprotein receptors) are involved in the molecular uptake into cells through the endocytic pathway as specific receptors. Some (for example, natural killer receptor NKR-P1) are involved in the signal transduction based on cell-cell recognition. Oligomerization (dimer, trimer or hexamer depending on the molecule) allows them to bind to carbohydrate ligands with multivalent interaction. The third type of transmembrane C-type lectin is type I transmembrane protein with tandem extracellular CRDs. Extracellular amino termini have a cysteine-rich domain followed by a fibronectin type II domain, tandem CRDs, transmembrane domain and carboxyl terminal cytoplasmic domain. This group includes cell surface mannose receptors on macrophages (and on other types of cells) and DEC-205, which is a cell surface molecule of dendritic cells (a major type of antigen presenting cells). These molecules are involved in the molecular uptake into cells. An interesting molecular feature of this group is that a tandem arrangement of CRDs produces an effect (capability of multivalent interaction with carbohydrate ligands) similar to the oligomeric arrangement of CRDs, because at least some of the CRDs have carbohydrate binding-activity. | ||||||||||||||
| Yasuyuki Imai (Department of Microbiology, University of Shizuoka School of Pharmaceutical Sciences) | ||||||||||||||
| ||||||||||||||
| Mar.15, 1998 | ||||||||||||||
| ||||||||||||||