| Galectin: Definition and History |  |
|
 |
A general name proposed in 1994 for a family of animal lectins (1). Galectins are defined as lectins having both galactose-binding ability and amino-acid sequences which characterize galectins. In general, galectins are soluble, and metal-independent in their activity. They also hold many features of cytoplasmic proteins, i.e., have no disulfide bridges, no sugar chains, no signal sequences, and in most cases their N-terminal amino acids are acetylated. However, their histological localization is diverse, not restricted in cytoplasm but also in nuclei, on cell surfaces and in extracellular spaces, depending on the galectin species. As regards secretion, little is known, but a speculative model has been presented in which a classical signal sequence is not required (2). Galectins show wide biological distribution not only in vertebrates but also in invertebrates, including nematodes, insects and sponges, and more recently, in the fungus mushroom, the galectins of which have been proved to possess galactose-binding activity. A wide variety of biological phenomena have been shown to be related to galectins, e.g., development, differentiation, morphogenesis, tumor metastasis, apoptosis, RNA splicing, etc. However, relatively little is known about the mechanism by which galectins exert these functions, particularly in terms of carbohydrate recognition.
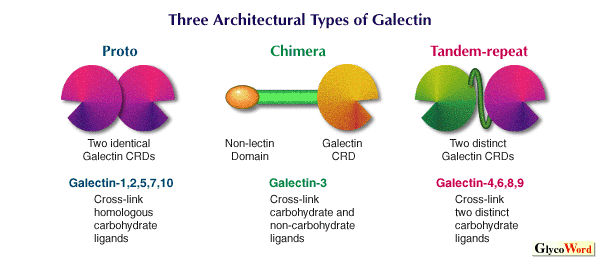
Galectins reported thus far can be classified into three types on the basis of structural architecture, i.e., proto, chimera and tandem-repeat types (see Fig.)(3). In 1994, it was proposed that mammalian galectins be numbered in the order of discovery (registration to GenBank). According to this system, more than 10 mammalian galectins have been registered. On the other hand, this numbering system cannot be applied directly to non-mammalian galectins, because it is difficult to correspond non-mammalian galectins to mammalian ones, and vice versa (in this regard, an expression like "nematode galectin-4" is irrelevant). | |
|
 |
|
|
The first galectin was discovered in 1975 by V. Teichberg et al. from extracts of electric organ/electric eel and other animal tissues as a low molecular weight (14-16 kDa) hemagglutinin, the activity of which is inhibitable with beta-galactodsides. After this investigation, a number of similar lectins (called "soluble b-galactoside-binding lectins" at the time) were identified in tissues from chick, bovine, human, rat, etc., and their biochemical properties and histological distributions were studied in relation to development and differentiation.
Since these lectins usually require a thiol-reducing reagent for the maintenance of their activity, K. Drickamer once designated them as "S-type" lectins ("S" stands for SH-requiring), from the viewpoint of C-type lectins ("C" stands for Ca-requiring). Though many scientists adopted this terminology, it was not always relevant. First, galectin-3 (discovered by J. Wang and called CBP35 those days; identical to Mac2 antigen) never requires a thiol-reducing reagent for the maintenance of activity. Second, P. Whitney et al. reported that galectin-1 became resistant to oxidative inactivation when galectin-1 was treated with a cysteine-modifying reagent, monoiodoacetamide. Finally, J. Hirabayashi et al., proved no cysteine residue is required for the sugar-binding function by means of site-directed mutagenesis, substituting one of 6 Cys with Ser with no change in sugar-binding activity. According to the present knowledge, a few reactive cysteine residues are critical for oxidative inactivation; these residues form abnormal disulfide bridge(s) accompanying drastic change in the three-dimensional structure, leading to inactivation.
| |
|
| Jun Hirabayashi (Teikyo University, Faculty of Pharmaceutical Sciences) | |
|
|
| References | | "Recent Topics on Galectins" (1997, ed. J. Hirabayashi) Trends Glycosci. Glycotechnol. 9 (45) |
| (1) | Barondes, S.H., et al.: Galectins: a family of animal b-galactoside-binding lectins. Cell 76, 597-598, 1994 |
| (2) | Cooper, D.N.W. and Barondes, S.H. : Evidence for export of a muscle lectin from cytosol to extracellular matrix and for a novel secretory mechanism. J. Cell Sci.110, 1681-1691, 1990 |
| (3) | Kasai, K. and Hirabayashi, J. : Galectins : a family of animal lectins that decipher glycocodes. J. Biochem. (Tokyo) 119, 1-8 |
| | |
| |
|
| Dec.15, 1997 |
|
| |
|
|
|
|



