

 |
 |
Mucin and Immune Response |
|||||||||||||||||
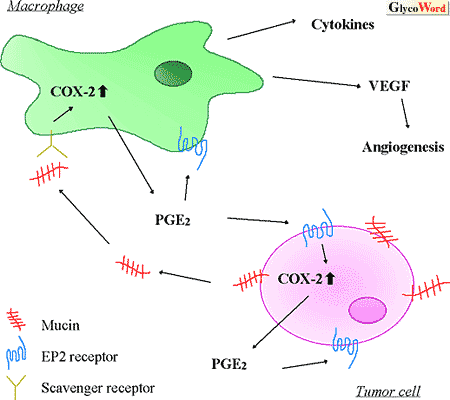
| Mucins have been investigated from two major points of view relevant to immune response: 1) immunotherapy of cancer patients and 2) effect on the immune system in the tumor-bearing state. Immunotherapy of cancer patients using mucins Monoclonal antibodies against tumor-associated carbohydrate antigens expressed on mucins have been used as a tool of diagnosis and immunotherapy. In the case of MUC1, which has been studied most extensively, underglycosylation of its core protein is usually observed with malignant transformation. It is known that cancer patients with MUC1-specific antibodies in their sera have a better prognosis. Recently, MUC1 has been investigated extensively as a target of cancer immunotherapy using cellular immune response. MUC1 is recognized by T cells in both MHC-unrestricted and –restricted manners. In the former case, CD3+, CD8+ MUC1-specific cytotoxic T lymphocytes (CTLs) were isolated from lymph nodes of pancreatic and breast cancer patients, and the MUC1 molecule was directly recognized by these CTLs. Among the latter cases, some contradictory data have been demonstrated on glycopeptide presentation by MHC class II. MUC1 isolated from ascites of patients with breast or pancreatic tumor was not processed by dendritic cells (DCs). Therefore, MHC-class II restricted T cells were not elicited. In contrast, Finn et al. demonstrated that MUC1 glycopeptides, which were produced by processing MUC1 glycoprotein endocytosed into DCs, were presented on MHC-class II without removing the carbohydrates, indicating the presence of a repertoire of glycopeptide-specific T cells. Immune response may be dependent on the site and structure of O-glycosylation. Since MUC1 in tumor cells has a different glycosylation profile from that in normal cells, the glycopeptide epitopes are expected to be tumor specific and useful for immunotherapy. It has also been revealed that glycopeptides processed from MUC1 could bind to MHC-class I molecule. A part of the glycosylated MUC1 tandem repeat, SAPD(T-GalNAc)RPA, could bind to MHC class I (H-2Kb) and the affinity was higher (~100 fold) than that of the unglycosylated peptide, SAPDTRPA. This glycopeptide-specific CTL could kill MUC1-expressing tumor cells. Effect of mucins on the immune system Generally, patients with MUC1-expressing tumors showed poorer survival rates than those with MUC1-negative tumors. MUC1 is known to suppress the killing activity of target cells by natural killer (NK) cells, lymphokine-activated killer (LAK) cells, and cytotoxic T cells (CTL), probably due to the rod-like molecule that sticks out from its cell surface. In addition, it has been reported that soluble MUC1 also suppressed target cell lysis by NK cells and T cell proliferative responses, but its mechanism remains to be elucidated. Recently, we found that mucins secreted from colon cancer cells activate monocytes and macrophages. We propose the cascade in the tumor microenvironment shown in the figure. Mucins are produced by cancer cells. Infiltrated macrophages are activated by the mucins through the scavenger receptor, resulting in overproduction of prostaglandin E2 (PGE2) by induction of cyclooxygenase 2 (COX-2). PGE2 secreted from the macrophages binds to the EP2 receptor present on cancer cells and/or other cells, and activated cancer cells and macrophages produce various factors including vascular endothelial growth factor (VEGF). It is also well known that PGE2 suppresses immune function and apoptosis. Thus, a series of reactions initiated by mucins seems to produce conditions in epithelial cancer tissues favorable for cancer cell growth. SAPDTRPA=Ser-Ala-Pro-Asp-Thr-Arg-Pro-Ala |
|||||||||||||||||
|
|||||||||||||||||
| Hiroshi Nakada (Kyoto Sangyo University, Faculty of Engineering) | |||||||||||||||||
|
|||||||||||||||||
| Oct.29 , 2004 | |||||||||||||||||
|
|
|||||||||||||||||
|
|||||||||||||||||