

 |
 |
C-type Lectins Expressed on Macrophages and Dendritic Cells |
|||||||||||||||||||||||
| (Update Issue: May.22, 2007) | |||||||||||||||||||||||
| Macrophages and dendritic cells are distributed in peripheral tissues throughout the body. They kill and eliminate invading foreign substances and are therefore responsible for innate immunity. They also trigger acquired immunity by capturing foreign antigens, migrating to secondary lymphoid organs, and presenting the antigens to T lymphocytes. Recently a number of C-type lectins specifically expressed on macrophages and dendritic cells have been identified (Table). Their involvement in the functions of macrophages and dendritic cells are being studied extensively. Structures of the lectins are diverse in their carbohydrate recognition domain (CRD) structure, number of CRDs in a molecule, orientation of the N-terminus, and amino acid sequence of the cytoplasmic region. Macrophages and dendritic cells consist of many subpopulations that differ in their distribution, differentiation stages, and progenitor cells. C-type lectins are utilized as suitable markers for the identification of a particular subpopulation such as langerin and BDCA-2. However, their ligand molecules have not been fully identified. Some of the known C-type lectins (L-selectin, etc.) are also expressed on the cell population. |
|||||||||||||||||||||||
|
|||||||||||||||||||||||
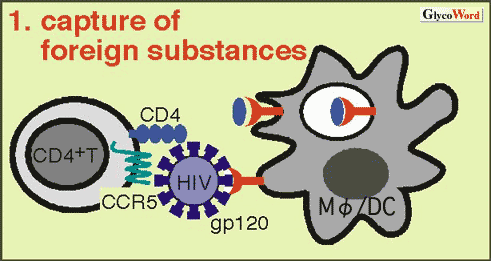
| 1. Capture of Foreign Substances (Fig.1) Many C-type lectins function as a receptor for capture of foreign substances. They contain unique motifs in their cytoplasmic regions, such as tyrosine motif and dileucine motif for trafficking to clathrin-coated vesicles, and acidic amino acid clusters for lysosome trafficking. A few C-type lectins such as DC-SIGN (dendritic cell-specific ICAM-3 grabbing non-integrin) and mannose receptor (MR) are known to be able to recognize a variety of pathogens. Viruses, including HIV, utilize the C-type lectins for successful infection. They initially bind to the lectins on dendritic cells in peripheral mucosal tissues, move to secondary lymphoid organs by utilizing the migratory capacity of the cells, and achieve infection to T lymphocytes. DC-SIGN was identified as a binding protein for gp120 of HIV envelope protein. This molecule as well as other lectins is thought to facilitate transinfection of HIV from dendritic cells to T lymphocytes. |
|||||||||||||||||||||||
 |
|||||||||||||||||||||||
|
Fig.1
|
|||||||||||||||||||||||
|
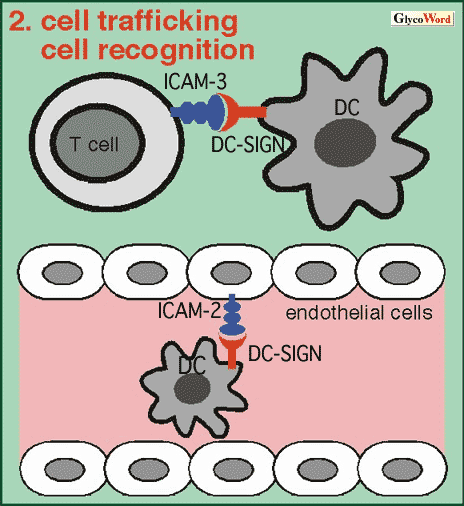
2. Cell Trafficking and Cell Recognition (Fig.2)
During the process of extravasation of dendritic cell precursors, interaction between DC-SIGN on dendritic cells and ICAM-2 on endothelial cells is reported to be important for the initial attachment. Adhesion between dendritic cells and T lymphocytes is important for antigen presentation. DC-SIGN was identified as a recognition molecule for ICAM-3 on T lymphocytes. The DC-SIGN and ICAM-3-mediated adhesion is involved in the initial interaction. |
|||||||||||||||||||||||
 |
|
||||||||||||||||||||||
|
Fig.2
|
|||||||||||||||||||||||
|
3. Signal Transduction
The amino acid sequence of the cytoplasmic region of the C-type lectins is diverse. Some C-type lectins contain motifs that are related to signal transduction and actually regulate cell functions. Zymosan derived from yeast cell wall is known to be an activating reagent for macrophages and dendritic cells. Recently dectin-1 was characterized as a receptor for C-type lectins also transfer inhibitory signals. Plasmacytoid dendritic cells produce a huge amount of interferon- |
|||||||||||||||||||||||
| Nobuaki Higashi (Graduate School of Pharmaceutical Sciences, University of Tokyo) |
|||||||||||||||||||||||
|
|||||||||||||||||||||||
| Apr.23 , 2004 | |||||||||||||||||||||||
|
|
|||||||||||||||||||||||
|
|||||||||||||||||||||||