|
Determination of Molecular Masses of Regularly Arranged Acidic Polysaccharides
|
|
|
 |
Hyaluronic acid (HA) and N-acetylneuraminic acid (NeuAc) polymers are polysaccharides composed of simple repeating units, although they show wide distributions in molecular masses (Fig. 1. ). |
|
|
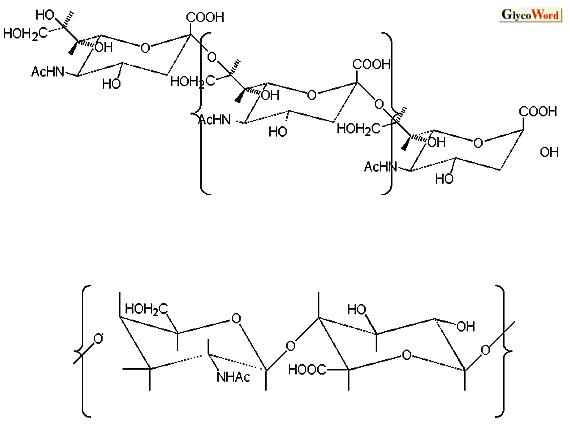 |
| Fig. 1. Structures of hyaluronic acid and N-acetylneuraminic acid polymers |
|
|
|
|
HA is a linear polysaccharide composed of a repeating, negatively charged disaccharide unit of glucuronic acid and N-acetylglucosamine. NeuAc polymers are polysaccharides composed of a NeuAc unit. The molecular mass of these polysaccharides, i.e. degree of polymerization, is closely related to their biological functions. For example, Finne and M el el reported that a large alpha-2,8-linked NeuAc oligomer (decasaccharide) was required for binding with antibodies of group B meningocca polysaccharides (1). Park et al. reported that HA oligomers composed of less than 10 – 12 saccharide units are resistant to hyaluronate lyase (2). reported that a large alpha-2,8-linked NeuAc oligomer (decasaccharide) was required for binding with antibodies of group B meningocca polysaccharides (1). Park et al. reported that HA oligomers composed of less than 10 – 12 saccharide units are resistant to hyaluronate lyase (2).
It is important to obtain the accurate molecular mass or distribution of the molecular species of polysaccharide for understanding their biological functions. Molecular mass of oligo- and polysaccharides and distribution of the molecular species can be determined by high-performance anion exchange chromatography (HPAEC) with pulsed-amperometric detection or high-performance capillary electrophoresis (CE) (3-5).
Separation of polysaccharides by HPAEC is based on the dissociation of hydroxyl groups under highly basic conditions. N-Acetylneuraminic acid polymers composed of at least one hundred monomer units (molecular mass, ca 30,000) were successfully separated with high resolution and high sensitivity. In the analyses by HPAEC, smaller oligomers are observed earlier and larger oligomers later.
Capillary electrophoresis is also a useful tool for high-resolution separation of N-acetylneuraminic acid polymers and hyaluronic acid. An example of the separation of hyaluronic acid is shown (Fig. 2. ) |
|
|
|
|
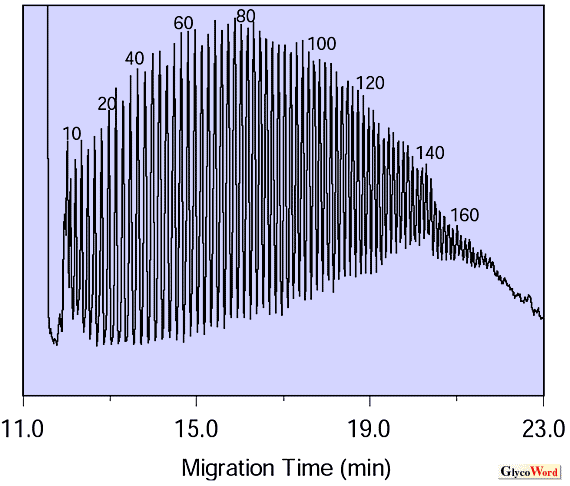 |
| Fig. 2. Separation of hyaluronic acid using capillary electrophoresis |
|
|
|
|
|
Hyaluronic acid composed of more than 100 disaccharide units can be distinguished. In this example, a running buffer containing polyethyleneglycol as an additive is employed using a chemically modified fused-silica capillary (20 cm length), the inner-surface of which is chemically immobilized with dimethylpolysiloxane. The capillary is commercially available for capillary gas chromatography. During electrophoresis, HA molecules move through the dynamic matrix formed by entanglement of polyethyleneglycol in the running buffer. Small molecules can pass through the matrix fast and large oligomers pass more slowly. This mode of separation is capillary electrophoresis version of SDS-polyacrylamide gel electrophoresis.
The molecular masses and distribution of molecular species obtained by these techniques will afford valuable information regarding their biological functions.
|
|
|
|
|
|
Kazuaki KakehiĀ@( Faculty of Pharmaceutical Sciences, Kinki University) |
|
|
|
|
|
| References |
(1) |
Finne J, M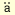 el el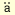 , PH : Cleavege of the polysialosyl units of brain glycoproteins by a bacteriophage endosialidase. Involvement of a long oligosaccharide segment in molecular interactions of polysialic acid. J. Biol. Chem. 260, 1265-1279, 1985 , PH : Cleavege of the polysialosyl units of brain glycoproteins by a bacteriophage endosialidase. Involvement of a long oligosaccharide segment in molecular interactions of polysialic acid. J. Biol. Chem. 260, 1265-1279, 1985
|
|
(2) |
Park Y, Cho S, Linhardt, RJ : Exploration of the action pattern of streptomyces hyaluronate lyase using high-resolution capillary electrophoresis. Biochim. Biophys. Acta, 1337, 217-226, 1997 |
|
(3) |
Zhang Y, Inoue, Y, Inoue S, Lee YC : Separation of Oligo/Polymers of 5-N-acetylneuraminic acid, 5-N-glycolylneuraminic acid, and 2-keto-3-deoxy-D-glycero-D-galacto-nononic acid by high-performance anion-exchange chromatography with pulse amperometric detector. Anal. Biochem. 250, 245 - 251,1997
|
|
(4) |
Stefansson M, Novotny MV : Modification of the electrophoretic mobility of neutral and charged polysaccharides. Anal. Chem. 66, 3466-3471, 1994
|
|
(5) |
Kakehi K, Kinoshita M, Oda Y : Capillary electrophoresis of N-acetylneuraminic acid: correlation between migration order reversa and biological functions. Anal. Chem. 71, 1592-1596 , 1999
|
|
|
|
|
|
| Sep.15, 1999 |
|
|
|
|
|
|
|



