|
Glycotechnology Using Endo-type Glycosidases
|
|
|
 |
Current problem in glycotechnology
The urgent problem of today’s biotechnology is the development of a method to synthesize carbohydrate chains having biological activities and to attach the carbohydrate chains to peptide or lipid. At present, it is very difficult to synthesize chemically long and branched carbohydrate chains of glycoconjugates: glycoproteins (N-glycan and O-glycan), proteoglycans and glycolipids. Especially there is no useful method for the attachment of carbohydrate chains to peptide and lipid, in order to solve the problem that some recombinant proteins produced by genetic engineering do not possess carbohydrate chains, which are an essential feature of native proteins. On the other hand, the ligase and glycosyltransferase for the reconstruction and attachment of the carbohydrate chains are not presently available.
Usefulness of endo-type glycosidase
The endo-type glycosidases hydrolyze glycoside bonds of the inner parts of the carbohydrate chains or of the linkage region between a core protein (or lipid) and a carbohydrate chain of glycoconjugates (Fig. 1). As a result, an oligosacaharide is released from the non-reducing terminal site of the carbohydrate chain. At the same time, the enzymes also catalyze translation reaction of the oligosaccharide, as an apparent reverse reaction.
Therefore, the transglycosylation reaction is very useful for the synthesis and reconstruction of carbohydrate chains and the attachment of carbohydrate chains to peptide and lipid of glycoconjugate (Fig. 2).
|
|
|
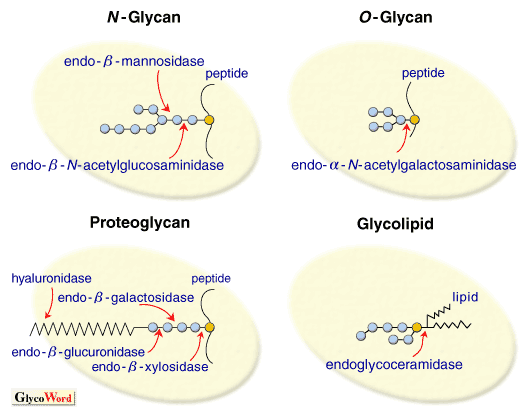 |
| Fig1. Endo-type Glycosidases Acting on Glycoconjugates |
|
|
|
|
| Fig2. Strategy of Glycoconjugate Synthesis Using Endo-type Glycosidases |
|
|
|
|
|
Endo-type glycosidases acting on glycoconjugates
There are two types of endo-type glycosidase: one acts on the linkage region between a carbohydrate chain and a core part (peptide or lipid) of glycoconjugates, and the other acts on the inner part of the carbohydrate chain apart from the linkage region.
|
|
|
| 1. N-glycan |
|
(1) |
Endo-beta-N-acetylglucosaminidase (acting on core structure part of carbohydrate chain of N-glycans) |
|
(2) |
Endo-beta-mannosidase (acting on core structure part of carbohydrate chain of N-glycans)
|
| 2. O-glycan |
|
(1) |
Endo-alpha-N-acetylgalactosaminidase (acting on linkage region between carbohydrate chain and peptide of O-glycan) |
| 3. Proteoglycan |
|
(1) |
Endo-beta-xylosidase, endo- beta-galactosidase and endo- beta-glucuronidase (acting on linkage region between glycosaminoglycan chain and core peptide of proteoglycan) |
|
(2) |
Testicular hyaluronidase, endo- beta-N-acetylhexosaminidase (acting on inner part of glycosaminoglycan chain: hyaluronic acid and chondroitin sulfate) |
| 4. Glycolipid |
|
(1) |
Endoglycoceramidase (acting on linkage region between carbohydrate chain and lipid of glycolipid)
|
|
|
|
Special features of synthesis and reconstruction of carbohydrate chains using endo-type glycosidases.
|
|
|
| (1) |
The enzyme is able to transfer an oligosaccharide block from a carbohydrate chain as a donor into a hydroxy group of carbohydrate, peptide or lipid as an acceptor. |
| (2) |
The reactions are in the regio- and stereo-selective transglycosylation. |
| (3) |
The reactions proceed under mild conditions in a short time.
|
|
|
|
It is predicted that the glycotechnology using endo-type glycosidases will make great progress in combination with chemical synthesis methods. |
|
|
|
Masahiko Endo (School of Medicine, Hirosaki University) |
|
|
|
|
|
| References |
(1) |
Takagaki K, Kon A, Kawasaki H, Nakamura T, Tamura S, Endo M. : Isolation and characterization of Patnopecten mid-gut gland endo-beta-xylosidase active on peptidochondroitin sulfate. J. Biol. Chem. 265, 854-860, 1990
|
|
(2) |
Takagaki K, Nakamura T, Izumi J, Saitoh H, Endo M. : Characterization of hydrolysis and transglycosylation by testicular hyaluronidase using ion-spray mass spectrometry. Biochemistry, 33, 6305-6507, 1994
|
|
(3) |
Saitoh H, Takagaki K, Majima M, Nakamura T, Matsuki A, Kasai M, Narita H, Endo M : Enzymic reconstruction of glycosaminoglycan oligosaccharide chain using the
transglycosylation reaction of bovine testicular hyaluronidase. J. Biol. Chem. 270, 3741-3747, 1995
|
|
|
|
|
|
| Jun. 15, 2001 |
|
|
|
|
|
|
|



