|
Intramolecular Aglycon Delivery for Stereoselective Synthesis of beta-Manno Glycoside
|
 |
|
 |
Intense research activities have been devoted to the development of methodologies for efficient O-glycoside bond-forming reactions. Modern glycosylation reactions utilize glycosyl donors such as glycosyl fluoride, thioglycoside and trichloroacetimidate, which are relatively stable yet can be easily activated once exposed to certain promoters. By making the appropriate choices in terms of leaving groups, activating reagents and reaction conditions, a wide variety of structural patterns found in glycan chains can be synthesized.
However, stereochemical problems in O-glycoside bond formation have not been solved in a general sense. Among various types of oligosaccharides of natural origin, beta-glycoside of D-mannose is considered to be the most challenging from the synthetic point of view. This difficulty arises from its unique stereochemical array at C-1/2 positions, i.e. the 1,2-cis relationship eliminates the use of neighboring group participation for stereochemical control. The formation of beta-manno glycoside is also disfavored for the stereoelectronic reason. On the other hand, the biological importance of beta-manno glycoside is quite obvious, since all types of asparagine (Asn)-linked glycoprotein oligosaccharides have a so-called "core" pentasaccharide which contains beta-configured mannose (beta-Man) linked to the 4-position of N-acetylglucosamine (GlcNAc). Therefore, in order to pursue synthetic approaches to glycoprotein and related molecules, stereocontrolled synthesis of beta-Man is of particular significance.
Although numerous approaches have been investigated to solve this problem, the majority can be put into one of two categories. Schematically, the most straightforward approach is direct glycosylation using a mannosyl donor. Since the formation of beta-manno glycoside is disfavored under ordinary conditions, a promoter with special properties in combination with a certain type of leaving group is required. General speaking, the predictable tactic has been the glycosylation-inversion approach, which consists of beta-gluco(1,2-trans) glycosylation followed by the inversion of C-2 stereochemistry by oxidation-reduction. Approaches based on O-alkylation of C-1 OH and enzymatic glycosylation have also been investigated.
A conceptually novel strategy called intramolecular aglycon delivery (IAD) has been demonstrated to be highly useful for stereoselective beta-mannosylation. The distinct feature of the IAD approach is the exclusive formation of the single isomer. Because of the stereochemical constraint, the formation of the alpha-isomer is strictly forbidden. This approach was first proposed and tested by Barresi and Hindsgaul in 1991. They prepared mixed isopropylidene acetal from thiomannoside isopropenyl ether and alcohol, which was transformed into beta-mannoside stereoselectively. Shortly after this, Stork and coworkers reported the use of a silaketal-like intermediate for the same purpose.
We developed a newer version of IAD where a mannosyl donor equipped with a p-methoxybenzyl (PMB) group at the C-2 position was used as the precursor of a tethered intermediate. Treatment of the mannosyl donor with aglycon in the presence of DDQ cleanly afforded the mixed acetal which served as a tethered intermediate for IAD. Subsequent activation of the mannose anomeric position triggered IAD to afford beta-mannoside as a single stereoisomer(1). Using this approach, it is now possible to prepare the structure that corresponds to Man beta1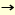 4GlcNAc in over 80% yield(2). Synthetic studies on naturally occurring glycan chains are now in progress in this laboratory(3). 4GlcNAc in over 80% yield(2). Synthetic studies on naturally occurring glycan chains are now in progress in this laboratory(3).
|
|
|
|
|
|
|
Yukishige Ito (RIKEN, The Institute of Physical and Chemical Research) |
|
|
|
|
|
| References |
(1) |
A, Dan, Y, Ito, T, Ogawa J. Org. Chem. 60, 4680-4681, 1995 |
|
(2) |
Y, Ito, Y,Ohnishi, T, Ogawa, Y, Nakahara Synlett, 1102-1104, 1998 |
|
(3) |
J, Seifert, M, Lergenmuller, Y, Ito Angew. Chem. in press |
|
|
|
|
|
| Dec. 15, 1999 |
|
|
|
|
|
|
|



