Selectivity of glycosyltransferases for substrate proteins | ||||||||||||||||||||||||||||||||
 |
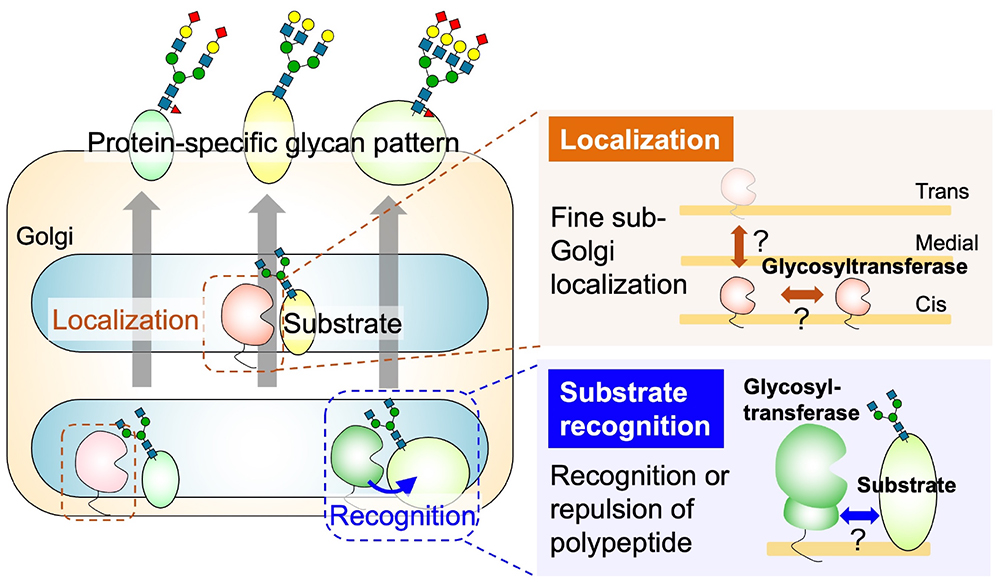
Many animal proteins are glycosylated, and they can be categorized based on the structure of their glycan and the amino acids to which they are attached. The four main classes are N-glycans, O-glycans (further subdivided into O-GalNAc, O-Man, O-Fuc, O-GlcNAc, and O-Gal based on the core structure), glycosaminoglycans (GAG), and glycosylphosphatidylinositols (GPI). It is widely recognized that individual proteins express different glycans. The mechanisms by which different glycan classes are attached to different proteins have been well studied. They are primarily determined by the enzyme-substrate protein recognition property, which catalyzes the initial step of glycan biosynthesis, namely the transfer of monosaccharides to amino acids. For example, the O-Fuc glycan modification depends on the recognition of the epidermal growth factor or thrombospondin type 1 repeat domains of the substrate protein by protein O-fucosyltransferases POFUT1 or POFUT2 (1). The anchoring of GPI depends on the recognition of the C-terminal GPI addition signal sequence by the GPI transamidase complex (2). Consequently, research has progressed into identifying the amino acid sequences and domains necessary for enzyme recognition in each glycan class. After the initial step, the elongation of sugar chains occurs; this requires glycosyltransferases to recognize the substrate oligosaccharides and transfer the monosaccharides to extend the chain. However, different enzymes exhibit intrinsic specificity toward the structures of nonreducing ends in glycans. For example, β1,3-glucuronosyltransferases (GlcAT) GlcAT-P and GlcAT-S, which transfer a glucuronic acid residue, have different reactivities toward three N-acetyllactosamine (Galβ1-4GlcNAc) moieties attached to the reducing end of a tri-antennary N-glycan (3). While it has been established that glycan profiles differ among proteins, the underlying mechanisms governing these variances are not fully elucidated. For example, immunoglobulin G (IgG) predominantly forms bi-antennary N-glycans, whereas erythropoietin predominantly forms tetra-antennary structures. This diversity in sugar chain structures is even found among proteins derived from the same cell. Moreover, the diversities are noted in O-GalNAc elongation, branching, sialylation, GAG length, and sulfate positioning as well as degree. The mechanisms governing the various terminal modifications of glycans (such as sialylation, fucosylation, and sulfation) for each protein also largely remain unclear. In cases in which glycans are highly specific to only a few proteins, glycosyltransferases recognize specific domains or motifs within those proteins to transfer the specific modifications. For example, polysialic acid at the N-glycan terminus is mostly attached to neural cell adhesion molecule (NCAM) because the biosynthetic enzymes ST8SIA2 and ST8SIA4 specifically recognize the acidic region of the fibronectin type III-like domain of NCAM (4). Recently, it was discovered that FUT9 recognizes specific amino acid sequences on LAMP1 that lead to the enhancement of its Lewis X modification (5). In case of the CoreM3 structure [GalNAc-β1-3GlcNAc-β1-4(P-6) Man-1-Thr/Ser] that is specifically expressed on α-dystroglycan (its impaired biosynthesis is associated with muscular dystrophy), the mechanism of glycan expression at the specific amino acids on α-dystroglycan involves the recognition of amino acid sequences near the CoreM3 glycans by POMGNT2 (7,8). Although the selection of proteins for glycosylation has been clearly observed through glycomics analysis, the specific factors driving differences in protein-specific glycan structures, such as N-glycan branching, terminal sialic acid modifications in N- and O-glycans, GAG sulfation, etc., remain largely unknown. The maturation of glycan structures on proteins occurs as they traverse the endoplasmic reticulum and Golgi apparatus, encountering various glycosyltransferases in a meticulously orchestrated secretory pathway. Therefore, the factors determining whether specific glycans are attached to a protein can be categorized by two aspects: how the enzymes and substrates encounter each other (a localization issue) and the mechanism of the sugar transfer reaction when they meet (a recognition issue). While other factors are also pivotal—such as enzyme expression levels, concentrations of sugar nucleotides as donor substrates, and folding states of substrate proteins—discussion in this chapter is primarily focused on the localization and recognition issues. Details relating to the localization of glycosyltransferases are provided in another chapter (see the “Glycosyltransferase localization (Coming soon)” section). In animal cells, glycosyltransferases are considered to be localized in specific zones within the cis, medial, and trans cisternae of the Golgi apparatus. Despite the critical role of enzyme localization in glycan synthesis (9), the mechanisms governing this localization remain unclear. Similarly, information concerning the recognition of substrate glycoproteins by enzymes is limited; traditional biochemical analyses have focused primarily on enzyme activity assays utilizing liberated oligosaccharides as substrates (10,11). However, given the contemporary technology facilitating advanced structural predictions, such as AlphaFold2 (12), it is now feasible to analyze the steric hindrances between individual glycoproteins and glycosyltransferases. Moreover, certain glycosyltransferases have been found to possess functional non-catalytic domains that are essential for the transfer of sugar to proteins (10,11). Hence, future investigations may explore whether mechanisms for substrate protein selection are based on recognition by non-catalytic functional domains as well as catalytic domains, which represents a potential novel avenue of inquiry in this field.
Yasuhiko Kizuka
|
|||||||||||||||||||||||||||||||
| References | |
|---|---|
| (1) | Holdener BC, Haltiwanger RS: Protein O-fucosylation: structure and function. Curr. Opin. Struct. Biol. 56, 78-86, 2019 |
| (2) | Kinoshita T: Biosynthesis and deficiencies of glycosylphosphatidylinositol. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 90, 130-143, 2014 |
| (3) | Yagi H, Yamada K, Ohno E, Utsumi M, Yamaguchi Y, Kurimoto E, Takahashi N, OKa S, Kawasaki T, Kato K: Development and Application of High Performance Liquid Chromatography Map of Glucuronyl N-glycans. Open Glycoscience 1, 8-18, 2008 |
| (4) | Sato C, Kitajima K: Polysialylation and disease. Mol. Aspects Med. 79, 100892, 2021 |
| (5) | Saito T, Yagi H, Kuo CW, Khoo KH, Kato K: An embeddable molecular code for Lewis X modification through interaction with fucosyltransferase 9. Commun. Biol. 5, 676, 2022 |
| (6) | Yagi H, Kuo CW, Obayashi T, Ninagawa S, Khoo KH, Kato K: Direct Mapping of Additional Modifications on Phosphorylated O-glycans of alpha-Dystroglycan by Mass Spectrometry Analysis in Conjunction with Knocking Out of Causative Genes for Dystroglycanopathy. Mol. Cell. Proteomics 15, 3424-3434, 2016 |
| (7) | Imae R, Kuwabara N, Manya H, Tanaka T, Tsuyuguchi M, Mizuno M, Endo T, Kato R: The structure of POMGNT2 provides new insights into the mechanism to determine the functional O-mannosylation site on alpha-dystroglycan. Genes Cells 26, 485-494, 2021 |
| (8) | Yang JY, Halmo SM, Praissman J, Chapla D, Singh D, Wells L, Moremen KW, Lanzilotta WN: Crystal structures of beta-1,4-N-acetylglucosaminyltransferase 2: structural basis for inherited muscular dystrophies. Acta Crystallogr. D Struct. Biol. 77, 486-495, 2021 |
| (9) | Welch LG, Munro S: A tale of short tails, through thick and thin: investigating the sorting mechanisms of Golgi enzymes. FEBS Lett. 593, 2452-2465, 2019 |
| (10) | Brockhausen I, Carver JP, Schachter H: Control of glycoprotein synthesis. The use of oligosaccharide substrates and HPLC to study the sequential pathway for N-acetylglucosaminyltransferases I, II, III, IV, V, and VI in the biosynthesis of highly branched N-glycans by hen oviduct membranes. Biochem. Cell Biol. 66, 1134-1151, 1988 |
| (11) | Takamatsu S, Korekane H, Ohtsubo K, Oguri S, Park JY, Matsumoto A, Taniguchi N: N-acetylglucosaminyltransferase (GnT) assays using fluorescent oligosaccharide acceptor substrates: GnT-III, IV, V, and IX (GnT-Vb). Methods Mol. Biol. 1022, 283-298, 2013 |
| (12) | Mirdita M, Schütze K, Moriwaki Y, Heo L, Ovchinnikov S, Steinegger M: ColabFold: making protein folding accessible to all. Nat. Methods 19, 679-682, 2022 |
| (13) | Osuka RF, Hirata T, Nagae M, Nakano M, Shibata H, Okamoto R, Kizuka Y: N-acetylglucosaminyltransferase-V requires a specific noncatalytic luminal domain for its activity toward glycoprotein substrates. J. Biol. Chem. 298, 101666, 2022 |
| (14) | Osada N, Nagae M, Nakano M, Hirata T, Kizuka Y: Examination of differential glycoprotein preferences of N-acetylglucosaminyltransferase-IV isozymes a and b. J. Biol. Chem. 298, 102400, 2022 |
Apr. 17, 2024