|
O-GlcNAc and Its Function
|
|
|
 |
Many nuclear and cytoplasmic proteins are glycosylated on
serine or threonine residue with a monosaccharide,  - -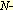 acetylglucosamine,
in an O-glycosidic linkage which is termed O-GlcNAc (Fig.
1). O-GlcNAc is present in all eukaryotes. O-GlcNAc modification
(O-GlcNAcylation) is one of the post-translational modifications and involved
in signal transduction (1). O-GlcNAc and O-phosphate alternatively occupy
the same or adjacent site. For example, the C-terminal domain of RNA polymerase
II, Thr58 of c-myc, which is a "hot spot" in mutation
for lymphomas, and Ser16 of estrogen receptor are modified
not only by O-GlcNAc but also a phosphate group. The half life of O-GlcNAc
is shorter than that of the polypeptides. O-GlcNAc modification is changed
dynamically by certain stimuli such as TPA, okadaic acid and others. Therefore,
it is suggested that one of the functions of O-GlcNAc is regulation of
transient phosphorylation. The glycosylation sites with O-GlcNAc have
no obvious consensus sequences. Unlike phosphorylation, O-GlcNAcylation
has not been observed in tyrosine residues. acetylglucosamine,
in an O-glycosidic linkage which is termed O-GlcNAc (Fig.
1). O-GlcNAc is present in all eukaryotes. O-GlcNAc modification
(O-GlcNAcylation) is one of the post-translational modifications and involved
in signal transduction (1). O-GlcNAc and O-phosphate alternatively occupy
the same or adjacent site. For example, the C-terminal domain of RNA polymerase
II, Thr58 of c-myc, which is a "hot spot" in mutation
for lymphomas, and Ser16 of estrogen receptor are modified
not only by O-GlcNAc but also a phosphate group. The half life of O-GlcNAc
is shorter than that of the polypeptides. O-GlcNAc modification is changed
dynamically by certain stimuli such as TPA, okadaic acid and others. Therefore,
it is suggested that one of the functions of O-GlcNAc is regulation of
transient phosphorylation. The glycosylation sites with O-GlcNAc have
no obvious consensus sequences. Unlike phosphorylation, O-GlcNAcylation
has not been observed in tyrosine residues.
|
|
|
 |
| |
| Fig. 1
Schematic diagram of O-GlcNAc modification for proteins. The protein
shown possesses a typical O-GlcNAc attachment site. However, the
consensus sequences to be modified is unknown. |
| |
| |
|
| |
Immunohistochemical analysis of the localization of O-GlcNAc
in rat pancreas revealed that O-GlcNAc is expressed on proteins in the
nucleus and cytoplasm of endocrine cells in the islets of Langerhans (2).
To date, a large number of cytoplasmic and nuclear proteins such as transcription
factors, nuclear pore proteins, oncogene products, tumor suppressors and
cytoskeletal proteins have been shown to be modified by O-GlcNAc. They
have two common features: (1) they are also phosphorylated and (2) they
form multimeric complexes with other proteins reversibly. Thus, the O-GlcNAcylation
might be a regulatory modification analogous to phosphorylation, involved
in signal transduction and protein multimerization.
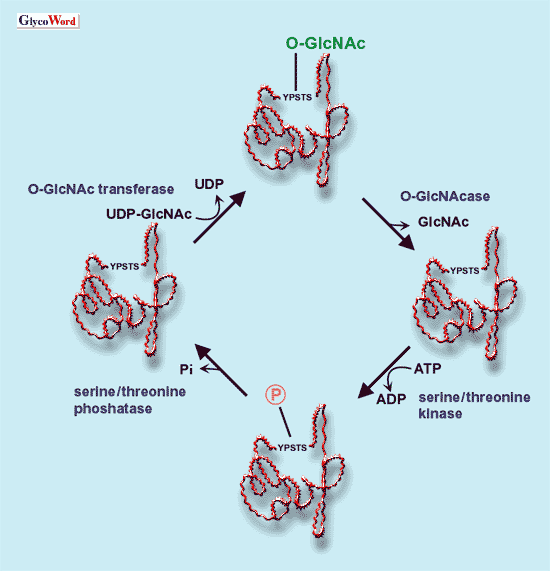
Enzymes for addition and for removal of the O-GlcNAc residue have been
purified and cloned (3, 4). UDP-GlcNAc: polypeptide O-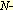 acetylglucosaminyltransferase
(O-GlcNAc transferase) adds acetylglucosaminyltransferase
(O-GlcNAc transferase) adds 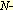 acetylglucosamine
to the hydroxyl group of serine or threonine residue(s) of proteins. The
O-GlcNAc transferase is itself modified by O-GlcNAc and the phosphate
group, and has 11 tetratricopeptide repeats (TPR) which are involved in
protein-protein interaction. Recently, using a yeast two-hybrid system
we identified GABAA receptor-associated protein (GRIF-1) and
its novel homolog, OIP106 as O-GlcNAc transferase interacting proteins(5).
O-GlcNAc transferase is abundant in brain and pancreas. Disruption of
O-GlcNAc transferase activity leads to the death of mouse embryonic stem
cells. This suggests that the O-GlcNAc modification is essential in cells.
O-GlcNAc specific acetylglucosamine
to the hydroxyl group of serine or threonine residue(s) of proteins. The
O-GlcNAc transferase is itself modified by O-GlcNAc and the phosphate
group, and has 11 tetratricopeptide repeats (TPR) which are involved in
protein-protein interaction. Recently, using a yeast two-hybrid system
we identified GABAA receptor-associated protein (GRIF-1) and
its novel homolog, OIP106 as O-GlcNAc transferase interacting proteins(5).
O-GlcNAc transferase is abundant in brain and pancreas. Disruption of
O-GlcNAc transferase activity leads to the death of mouse embryonic stem
cells. This suggests that the O-GlcNAc modification is essential in cells.
O-GlcNAc specific 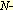 acetylglucosaminidase
(O-GlcNAcase) removes O-GlcNAc residues from proteins. O-GlcNAcase is
a cytosolic neutral acetylglucosaminidase
(O-GlcNAcase) removes O-GlcNAc residues from proteins. O-GlcNAcase is
a cytosolic neutral  - -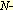 acetylglucosaminidase,
unlike the acidic lysosomal hexosaminidases. The regulation of O-GlcNAcylation
by these two enzymes is analogous to that of phosphorylation by kinases
and phosphatases (Fig. 2). acetylglucosaminidase,
unlike the acidic lysosomal hexosaminidases. The regulation of O-GlcNAcylation
by these two enzymes is analogous to that of phosphorylation by kinases
and phosphatases (Fig. 2).
There is a growing body of evidence that the aberrant O-GlcNAc modification
is correlated with diabetes, tumorigenesis, Alzheimer's disease (1, 6-9).
More detailed studies are necessary for determining the functions of O-GlcNAc
in a variety of biological systems.
|
|
|
 |
| |
Fig. 2
Reciprocal relationship between phosphorylation and O-GlcNAc modifications
of hydroxyl group of serine or threonine residue. O-GlcNAc: O-linked
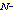 acetylglucosamine.
O-GlcNAcase: O-GlcNAc specific acetylglucosamine.
O-GlcNAcase: O-GlcNAc specific 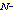 acetylglucosaminidase. acetylglucosaminidase. |
| |
| |
|
|
|
Yoshihiro Akimoto
(Department of Anatomy, Kyorin University School of Medicine) |
|
|
|
|
|
| References |
(1) |
Wells L, Vosseller K, Hart GW (2001) Glycosylation of nucleocytoplasmic
proteins: Signal transduction and O-GlcNAc. Science 291:2376-2378. |
|
(2) |
Akimoto Y, Kreppel LK, Hirano H, Hart GW (1999) Localization
of the O-GlcNAc transferase in rat pancreas. Diabetes 48:
2407-2413. |
|
(3) |
Kreppel LK, Blomberg MA, Hart GW. (1997) Dynamic glycosylation
of nuclear and cytosolic proteins. Cloning and characterization
of a unique O-GlcNAc transferase with multiple tetratricopeptide
repeats. J Biol Chem 272: 9308-9315. |
|
(4) |
Gao Y, Wells L, Comer FI, Parker GJ, Hart GW (2001) Dynamic O-glycosylation
of nuclear and cytosolic proteins: cloning and characterization
of a neutral, cytosolic beta-N-acetylglucosaminidase from human
brain. J Biol Chem 276: 9838-9845. |
|
(5) |
Iyer SP, Akimoto Y, Hart GW (2003) Identification and cloning
of a novel family of coiled-coil domainÅ proteins that interact
with O-GlcNAc transferase. J Biol Chem 278:
5399-5409. |
|
(6) |
Akimoto Y, Kreppel LK, Hirano H, Hart GW (2000) Increased O-GlcNAc
transferase in pancreas of rats with streptozotocin-induced diabetes.
Diabetologia 43:1239-1247. |
|
(7) |
Akimoto Y, Kreppel LK, Hirano H, Hart GW (2001) Hyperglycemia
and the O-GlcNAc transferase in rat aortic smooth muscle cells:
elevated expression and altered patterns of O-GlcNAcylation. Arch
Biochem Biophys 389:166-175. |
|
(8) |
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic
complications. Nature 414: 813-820. |
|
(9) |
Vosseller K, Wells L, Lane MD, Hart GW (2002) Elevated nucleocytoplasmic
glycosylation by O-GlcNAc results in insulin resistance associated
with defects in Akt activation in 3T3-L1 adipocytes. Proc
Natl Acad Sci USA 99:5313-5318. |
|
|
|
|
|
| Jan. 22, 2003 |
|
|
|
|
|
|
|



