 |
Gangliosides are sialic acid-containing glycolipids mainly
present on the extracellular surface of cell membrane. Gangliosides on
the cell surface can be recognized by proteins on the other cells (trans-recognition)
or by proteins on the same cells (cis-recognition). At present,
it is not established that which type of interaction is physiologically
more important. Here, I would like to introduce the oligosaccharide moiety
dependent-inhibitory effect of gangliosides on cell surface-enzyme as
an example of cis-interaction between gangliosides and proteins.
Enzymes having catalytic sites on their extracellular domains are called
as ecto-enzymes. Lymphocyte cell surface antigen CD38 is a single transmembrane
protein with molecular mass of 46 kDa and an ecto-enzyme of NAD glycohydrolase.
Since CD38-deficient mice show complete loss of tissue-associated NADase
activity, CD38 represents a major NADase in mammalian cells. The NADase
activity of the extracellular domain of CD38 is inhibited by various gangliosides
species. The inhibitory effect depends on the structure of the oligosaccharide
moiety. b-series gangliosides having tandem sialic acid residues linked
to the internal galactose residue (GT1b, GQ1b, GQ1b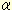 )
are especially potent inhibitors (1). The negative charges in the carboxyl
groups of the sialic acid residue are important. Thus, we have proposed
that the two carboxyl groups of the tandem sialic acid residues mimic
the diphosphate moiety of NAD+ and that in this way b-series gangliosides
act as inhibitors of NAD-metabolizing enzymes (2). )
are especially potent inhibitors (1). The negative charges in the carboxyl
groups of the sialic acid residue are important. Thus, we have proposed
that the two carboxyl groups of the tandem sialic acid residues mimic
the diphosphate moiety of NAD+ and that in this way b-series gangliosides
act as inhibitors of NAD-metabolizing enzymes (2).
After incubation of the CD38-expressing cells with GT1b, NADase activity
is inhibited. The time course of inhibition is slower than that of the
incorporation of GT1b into the cells, suggesting that incorporation into
the cell membrane is a prerequisite for inhibition. Inhibition occurred
efficiently when GT1b and CD38 are present on the cell surface rather
than on different cells. The inhibitory effect of GT1b is more potent
than that of GD1a. Accordingly, it is considered that CD38 recognizes
the oligosaccharide moiety ofGT1b on the same cell surface.
The oligosaccharide moiety of gangliosides is present within 20-25 above the cell surface, much closer than that on glycoproteins. Investigation
of protein recognition mechanism specific to the oligosaccharide moiety
of gangliosides will contribute to a better understanding of the role
of glycoepitopes very close to the cell suface.
above the cell surface, much closer than that on glycoproteins. Investigation
of protein recognition mechanism specific to the oligosaccharide moiety
of gangliosides will contribute to a better understanding of the role
of glycoepitopes very close to the cell suface. |
|
|
| References |
(1) |
Hara-Yokoyama M., Kukimoto I., Nishina H., Kontani K., Hirabayashi
Y., Irie F., Sugiya H., Furuyama S., Katada T., J.Biol.Chem.
271, 12951-12955, 1996 |
|
(2) |
Hara-Yokoyama M., Hirabayashi Y., Irie F., Shuto B., Moriishi
K., Sugiya H., Furuyama S.,J. Biol. Chem. 270,
8115-8121, 1995 |
|
(3) |
Hara-Yokoyama M., Nagatsuka Y., Katsumata O., Irie F., Kontani
K., Hoshino S., Katada T., Ono Y., Fujita-Yoshigaki J., Sugiya H.,
Furuyama S., Hirabayashi Y.,Biochemistry, 40,
888-895, 2001 |
|
|
|
|
|



