

 |
 |
Molecular diversity and functions of glycosylated lipids in biological membranes |
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (First version published:Jun.15, 2002) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
Glycolipids are present ubiquitously in cell surface membranes (1). The most common glycolipids in mammals are a class of sphingolipids, glycosphingolipids (GSLs). Major GSLs including gangliosides are derived from glucosylceramide (GlcCer) that is synthesized by a specific glycosyltransferase, ceramide glucosyltransferase (GlcT-1/GCS/UGCG). Galactosylceramide (GalCer) formed by ceramide galactosyltransferase (CGT) is also present as a dominant glycolipid in myelin sheath. This class of glyceroglycolipids is found in particular tissues: galactosylalkylacylglyceride (seminolipid) in the testis. Cholesterol is also modified by glucose and glucuronic acid, which are found in human fibroblast and liver, respectively (Table 1). |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Recent biological cell studies show that GSLs in cell membranes are preferentially distributed into lipid domains, so-called rafts (see also “Glyco Word” by Higashi). Raft-like lipid microdomains can be isolated biochemically as a low density Triton X-100 insoluble fraction. The fraction isolated contains sphingolipids (GSLs and sphingomyelin) and cholesterol. Importantly, the glycolipid-enriched membrane domain also includes proteins related to signal transduction such as src-family kinases. The lipid domains are suggested to play roles in cell-cell adhesion and receptor-mediated signal transduction. A glycolipid-enriched lipid microdomain is also involved in targets for host pathogens (Vibrio cholera, O157, HIV) and their toxin bindings. The lipid raft hypothesis is still a matter of debate. Nonetheless, the biochemical approach has revealed a new glycosylated phospholipid, phosphatidylglucoside (PtdGlc)(Fig. 1). Very interestingly PtdGlc is exclusively composed of saturated fatty acids (C18:0/C20:0)(2). The biological function of PtdGlc remains to be elucidated. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
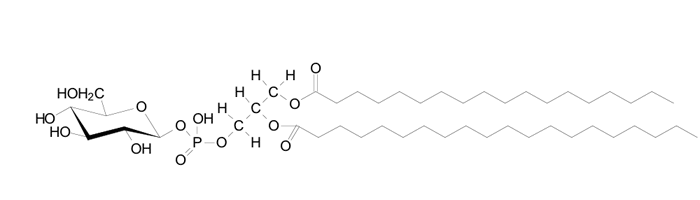 Fig.1 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A cell line deficient in an entire group of GSLs was established from B16 mouse melanoma. This mutant cell line is defective in a GlcCer synthase activity. In vitro studies on glycolipid function using the mutant cells show that the glycolipid biosynthesis is not essential for cell survival and proliferation, at least in the melanoma cells. Sphingomyelin as the sole sphingolipid on the plasma membrane can act as a substitute. Similarly, GSLs are not a critical component for membrane domain formation. These results from in vitro experiments indicate that GSLs apparently do not have house-keeping functions when studied at the single cell level. Thus, GSL research is now focusing on multicellular systems or whole animals. To solve the riddle of glycolipid functions, a method to eliminate a gene of glycolipid glycosyltransferase in mice has been developed using a homologous recombination system and several knockout mice have been generated (Table II). Surprisingly, mutant mice deficient in complex gangliosides are born normally, showing no apparent abnormalities. Even a double knockout mouse of GD3 synthase and GM2/GD2 synthase, expressing only GM3 ganglioside, results in normal development. Studies with these mutant mice prove that complex gangliosides are not involved in cellular differentiation but rather in the maintenance (homeostasis) and regeneration of nervous system tissues (see “Glyco Word” by Furukawa). Very recently, Simpson et al. identified an autosomal recessive infantile-onset symptomatic epilepsy syndrome as GM3 synthase deficiency (3). In the case of mouse, GM3 synthase knockout causes enhanced insulin sensitivity (4). In 1999, a mutant mouse for GlcCer synthase (UGCG) was generated. Since the mutant mouse is deficient in all GSLs except for GalCer, it gives us valuable information on the general functions of GSLs (see “Glyco Word” by Hirabayashi & Ichikawa). GalCer knockout mouse was first generated in 1996. In contrast to the UGCG mutant, the CGT knockout mouse is born normally and exhibits no obvious abnormalities in myelin structure, although GalCer and sulfatide, both enriched in myelin, are lacking. However, the mouse shows severe generalized tremor and mild ataxia, proving that the GSLs play a role in the insulative function of myelin. More careful examination revealed that the phenotypes are due to abnormalities in the paranodal region. The same mouse shows that CGT is also essential for sperm development. As shown above, glycolipid research is now regarded as a potent and promising research target, especially for brain science of the post-genomic era. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Yoshio Hirabayashi (Hirabayashi Research Unit, Brain Science Institute, RIKEN) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mar. 19, 2007 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||