

 |
 |
UDP-glucose:ceramide glucosyltransferase |  | ||||||||||||||||
| (Update Issue: March.2, 2007) | |||||||||||||||||
 |
Ceramide glucosyltransferase (GlcT-1, EC 2.4.1.80) catalyzes the initial glycosylation step of glycolipid biosynthesis. Since over 400 different glycolipids are derived from glucosylceramide (GlcCer), GlcT-1 is an extremely important glycosylation enzyme. It was necessary to purify GlcT-1 protein and to clone and eventually sequence the gene encoding it. GlcT-1 was originally described by Basu et al in embryonic chicken brains(1). It has been difficult to purify this enzyme mainly because the enzyme is tightly membrane-bound and a qualitatively minor component of the Golgi membrane. In 1996, Paul et al. succeeded in purification of GlcT-1 protein up to 10,000-fold from the Golgi membrane of rat liver (2). The purified protein proved that the enzyme is highly specific to D,L erythro-ceramide but not for threo-ceramide. The enzyme required dioleoyl phosphatidylcholine for optimal activity. UDP-Glc was the preferred hexose donor. The unique feature of GlcT-1 is its membrane topology. Several research groups suggested that the synthesis of GlcCer occurred at the cytosolic side of the Golgi membrane, while other glycosylation enzymes involved in glycolipid synthesis take place at the lumenal side of the membrane. This means that GlcCer once formed must be translocated to the lumenal side of the Golgi apparatus for further glycosylation. Translocation of GlcT-1 is presumably facilitated by a protein factor, flippase. | ||||||||||||||||
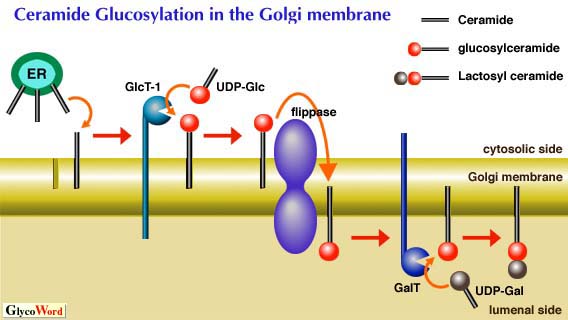 GlcT-1 : Glucosylceramide synthase, GalT : Lactosylceramide synthase | |||||||||||||||||
|
In 1996, a cDNA for human GlcT-1 was isolated by expression cloning using mouse melanoma GM-95 cells deficient in GlcT-1 activity (3). GlcT-1 cDNA proves that GlcT-1 is a totally novel protein. The predicted sequence of GlcT-1 has a type III membrane protein with N-terminal signal-anchor sequence and a long cytoplasmic C-terminal tail, which is consistent with previous reports that ceramide glucosylation occurred at the cytosolic surface of the Golgi apparatus. Chromosomal GlcT-1 gene is isolated from human and mapped at 9q31. Glycolipids derived from GlcCer are ubiquitous membrane lipid molecules, suggesting that GlcT-1 is widely distributed in mammalian tissues. In fact, Northern blot analysis shows that GlcT-1 mRNA is expressed in all human tissues examined (brain, heart, muscle, liver, skeletal muscle, placenta, etc.). Database searches reveal similar sequences in mouse, rat, C. elegans, cyanobacteria, and Drosophila melanogaster (fruit fly), indicating the lipid glucosylation is of biological importance(4). To gain insight into the role of ceramide glucosylation in whole animal, it is necessary to create knock-out mice deficient in GlcT-1. Since GlcCer is a central lipid as a precursor for numerous glycolipids including brain gangliosides, the mice would be invaluable tools for understanding of the general functions of glycolipids. | |||||||||||||||||
| Yoshio Hirabayashi and Shinichi Ichikawa (Laboratory for Cellular Glycobiology, The Institute of Physical and Chemical Research, RIKEN) | |||||||||||||||||
| |||||||||||||||||
| Jun.15, 1998 | |||||||||||||||||
| |||||||||||||||||