| GM3 synthase | |
|
 |
Ganglioside GM3 is a common precursor of the major ganglio-series ganglio-sides, and is distributed in almost all mammalian tissues. GM3 is synthesized by the transfer of sialic acid from CMP-sialic acid to non-reduced terminal galactose residue of lactosylceramide through the alpha 2,3 glycosyl bond, and the reaction is catalyzed by GM3 synthase (CMP-NeuAc:lactosylceramide alpha 2,3-sialyltransferase: EC 2.4.99.9). The enzyme is active at the branch point of the extension of the sugar chain on glycosphingolipids, and the regulatory expression of SAT-I activity is considered to affect the biosynthesis not only for ganglio-series but also for lacto/neolacto-,globo- and/or isogloboseries glycosphingolipids.
| |
|
|
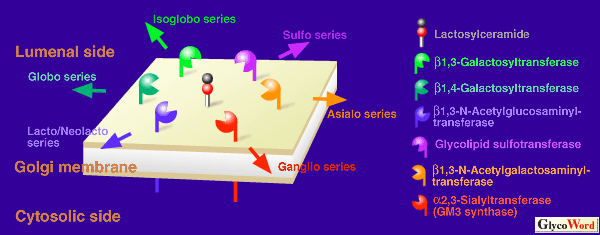
Fig.1 GM3 synthase competes with four other glycosyltransferases and one sulfotransferase for a common substrate, lactosylceramide. Expression of each transferase is regulated developmentally and spatially in the process of embryogenesis, differentiation and carcinogenesis, resulting in proper composition of glycosphingolipids in each tissue.
|
|
|
|
In 1991, Melkerson-Watson and Sweeley reported that SAT-I was enriched to 43,000-fold from rat liver, and in 1993, the enzyme was purified to 7,200-fold from rat brain by Preuss et al. It is unclear whether the two purified enzymes are the same protein, since they showed differences in molecular weight, substrate specificity, etc. Gu et al. proposed the possibility that SAT-I activity might be regulated through phosphorylation/dephosphorylation mechanisms.
One of the biological functions of GM3 is the differentiation induction of certain cells. During the monocytic differentiation of HL-60 cells with phorbol ester as the differentiation inducer, the level of GM3 is dramatically enhanced with a concomitant increase in GM3 synthase activity. Furthermore, the treatment of HL-60 cells with GM3 induced differentiation into monocytoid, as shown in the treatment of HL-60 cells with phorbol ester. In 1997, the SAT-I gene was cloned by a modified expression cloning using the monocytic differentiation system of HL-60 cells. SAT-I is a novel protein belonging to the sialyltransferase family (STs), although an invariant aspartic acid in sialylmotif L of all other sialyltransferases is replaced by histidine. This amino acid substitution is found in mouse and rat SAT-Is.
Unlike other sialyltransferases using glycosphingolipids as sialic acid acceptor, the substrate specificity of sat-I product was found to be highly restricted to lactosylceramide. This result seems to be incompatible with those of purified SAT-I described above. The expression of SAT-I mRNA shows a tissue- and species-specific pattern, although distribution of GM3 is ubiquitous in various tissues of animals. In human, brain, skeletal muscle and testis express high levels of the transcript of SAT-I gene, whereas low levels of the mRNA were found in liver and kidney. In mouse, the highest level of the mRNA was detected in liver, while the lowest was found in the kidney. On the other hand, kidney, as well as heart, brain, and spleen, express high levels of the mRNA in rat.
It is expected that studies on biological functions of GM3 have been stimulated by the cloning of the SAT-I gene. It would be interesting to investigated the origin of GM3 in an attempt to elucidated its biological significance in animals using the SAT-I gene as a clue.
| |
|

|
Fig.2 Schematic structure of human GM3 synthase. GM3 synthase belongs to sialyltransferase family with a transmembrane portion at NH2-terminal, a large lumenal catalytic domain with two conserved regions, so called sialylmotifs, while an invariant aspartic acid in the sialylmotif Ls of all other sialyltransferases is replaced by histidine in GM3 synthase (indicated by yellow letter). Red letters indicate amino acids conserved in sialyltransferases from mammalian sources. TM is transmembrane portion, and triangles show a potential N-glycosylation sites.
|
|
|
| Atsushi Ishii, Msaki Saito (National Cancer Center Research Institute, Virology Division) | |
|
|
| References | (1) | Melkerson-Watson, LJ, Sweeley, CC, J. Biol. Chem. 266, 4448-4457, 1991 |
| (2) | Preuss, U, Gu, X, Gu, T, Yu, RK, J. Biol. Chem. 268, 26273-26278, 1993 |
| (3) | Gu, X, Preuss, U, Gu, T, Yu, RK, J. Neurochem. 64, 2295- 2302, 1995 |
| (4) | Nojiri, H, Takaku, F, Terui, Y, Miura, Y, Saito, M, Proc. Natl. Acad. Sci. USA, 83, 782-786, 1986 |
| (5) | Ishii, A et al. J. Biol. Chem. 273, in press, 1998 |
| | |
| |
| Dec.15, 1998 |
|
| |
|
|
|
|



