| Sialylation of Glycolipids | |
|
 |
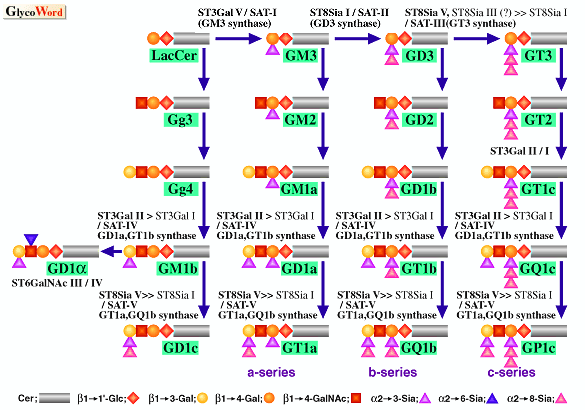
By means of expression cloning using ganglioside-specific antibody and PCR cloning using conserved motif "sialylmotif" which all sialyltransferases have various kinds of sialyltransferase genes involved in the sialylation of ganglioside have been cloned. Proposed biosynthetic pathways for gangliosides and each corresponding sialyltransferases are shown in the figure (1).
| |
|
|
Figure Legend Four kinds of ganglioside biosynthetic pathways, the a-series (comprising GM3, GM2, GM1, GD1a and GT1a), the b-series (comprising GD3, GD2, GD1b,and GT1b, and GQ1b), the c-series (comprising GT3, GT2, GT1c, GQ1c, and GP1c), and the a-series (comprising GD1a) , and the corresponding sialyltransferase genes are shown here.
|
|
|
|
Some terminal glycan structures in gangliosides are also contained in some glycoproteins. For example, Gal beta1,3GalNAc residue is contained in Gg4Cer, Gal beta1,3GalNAc beta1,4Gal beta1,4Glc1,1'Cer as well as in the O-linked glycan chains of glycoproteins such as asialofetuin. Two kinds of sialyltransferases, ST3Gal I and ST3Gal II, are capable of utilizing this Gal beta1,3GalNAc structure as acceptor substrate. By comparing their Km values for corresponding substrates, it is shown that asialofetuin serves as the predominant acceptor for ST3Gal I, whereas Gg4Cer is preferred to asialofetuin by ST3Gal II. Another example is the case of the Sia alpha2,3Gal beta1,3GalNAc structure which is present in both GM1b and the O-linked glycan chain of glycoproteins such as fetuin. The activity of ST6GalNAc III toward GM1b is stronger than that toward fetuin. On the other hand, ST6GalNAc IV prefers fetuin to GM1b. Taken together, even though there are sometimes two or more than two sets of sialyltransferases which recognize the same carbohydrate structure, their preferences for acceptor substrates seem to be different; some prefer glycoprotein to gangliosides as acceptor substrate, and others prefer glycolipids instead.
The different expression level of the sialyltransferase gene in the cells can sometimes lead to different conclusions. Even though GT3 synthase (SAT-III, ST8Sia V) is reported to be the best candidate sialyltransferase that is most likely involved in GT3 synthesis, there is a report that overexpression of GD3 synthase (ST8Sia I) cDNA leads to new expression of GT3.
| |
|
| Shinobu Kitazume-Kawaguchi (RIKEN Institute) | |
|
|
| Reference | (1) | Tsuji, S: Molecular cloning and functional analysis of sialyltransferases. J. Biochem. 120, 1-13, 1996 |
| | |
| |
| Dec.15, 1998 |
|
| |
|
|
|
|



