| Carbohydrate-Carbohydrate Interaction of Glycosphingolipids | |
|
 |
Since dramatic changes in surface carbohydrates during oncogenesis have been demonstrated, it is assumed that cell surface carbohydrates may play a role in cell-cell or cell-substrate recognition. Many models for cell-cell interaction based on carbohydrates have been proposed. In most of them, the carbohydrate bind to selectins, galectins and other carbohydrate-binding proteins through carbohydrate-protein interaction. In 1989, Hakomori and co-workers proposed an alternative model for carbohydrate-dependent cell adhesion, i.e., carbohydrate-carbohydrate interaction may initiate cell-cell interaction.
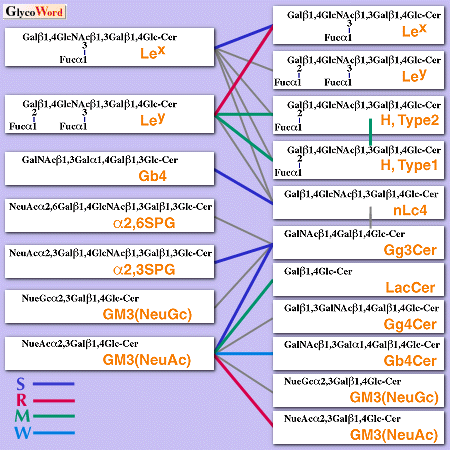
Preliminary evidence for carbohydrate-carbohydrate interaction came from studies including liposomes containing highly purified glycosphingolipids (GSLs). The following observations suggested that the specific GSLs bind to the complementary GSLs through interaction between their carbohydrate moieties: (a) Phosphatidylcholine-[14C]cholesterol liposomes containing a GSL bound to plates coated with the specific (complementary) GSL but not to those coated with other GSLs. The interaction was dependent on the GSL density in liposomes or on plates. (b) Beads coated with GSLs showed strong aggregation when beads coated with a GSL and those coated with the complementary GSL were mixed. The aggregation rate was dependent on the amount of the GSL on the bead surface. (c) The radiolabeled synthetic multivalent oligosaccharide bound to the complementary GSL adsorbed column and eluted with EDTA. (d) Finally, cells highly expressed a GSL specifically bound to the cells expressed the complementary GSL as well as plates coated with the complementary GSL. Fig. 1 summarizes the possible GSL-GSL interactions demonstrated in the systems described above. For example, Lex- Lex interaction was first discovered as the specific carbohydrate-carbohydrate interaction by Hakomori and co-workers. Lex-expressing cells (e.g., F9 cells) or cells in which Lex-GSL was exogenously incorporated were shown to aggregate in the presence of divalent cations. Mouse melanoma B16 cells, which strongly express GM3 adhered strongly to Gg3Cer, moderately to LacCer, and weakly to Gb4Cer as in the case of GM3-containing liposomes. B16 cells can adhere to Gg3Cer-expressing mouse lymphoma L5178 clone AA12 cells and endothelial cells which express LacCer. These cell adhesions and aggregations could be inhibited in the presence of liposomes containing the corresponding GSLs, the corresponding oligosaccharides, and antibodies against the corresponding GSLs, but not in the presence of simple liposomes, other oligosaccharides, or antibodies against other GSLs.
| |
|
 |
|
Fig. 1
Possible GSL-GSL interaction observed using GSL liposomes. S, M, W,and R indicate strong, moderate, weak and repulsive interaction, respectively. Gray line indicates no interaction. | |
|
|
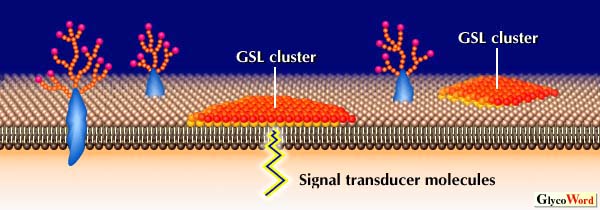
Some GSL-GSL interactions shown in Fig. 1 have been confirmed by measurement of molecular force or by a study with ion spry mass spectrometry. However, direct binding between the carbohydrate moieties of GSL by a study with NMR has not yet been demonstrated. Since GSL-containing liposome binding to GSL coated plates was dependent on GSL density in liposomes and on plates, multivalency or clustering of GSL is essential for GSL-GSL interactions. Tillack et al. observed from freeze-etch electron microscopic studies that GSLs are clustered at the cell surface or at the liposome surface. The phase separation of GSLs from matrix phospholipids was confirmed by measuring surface pressure-area isotherms of the mixed monolayer of GSL and synthetic phospholipids. Therefore, GSL on cell surface probably exists as large clusters to form microdomains (GSL-enriched microdomains). Such GSL clusters may be recognized very specifically by not only lectins and antibodies but also by clusters of complementary GSL (Fig. 2). Adhesion of cells or GSL-containing liposomes on GSL-coated plates may well be based on interaction between GSL clusters. In addition, GSL-GSL interaction is a rapid process, as compared with protein-protein interaction, although the strength of the interaction is weaker than that of protein-protein interaction. A synergistic effect between cell adhesion based on GSL-GSL interactions and adhesion based on integrins was also observed. Thus, GSL-GSL interaction may define the initial specificity and direction of the cell recognition, and regulate the following adhesion process.
| |
|
 |
|
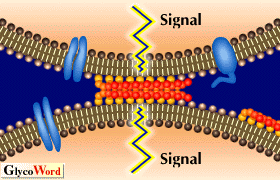
Fig. 2
Hypothetical scheme for organization of GSLs at cell surface and the possible cell adhesion events through GSL-GSL interaction. |
|
|
|
It was demonstrated that not only adhesion but also spreading and motility of cells expressing a GSL are enhanced on plates coated with the complementary GSLs. Recently, GSL-enriched microdomains have been shown to be enriched in several signal transducer molecules, e.g., c-Src, Ras, Lyn, and focal adhesion kinase (FAK). In addition, it was shown that tyrosine phosphorylation in FAK and GTP loading on Ras increased when cells highly expressing a GSL were adhered to the plates coated with the complementary GSL through carbohydrate-carbohydrate interaction (for example, in the case of B16 cell adhesion to Gg3-coated plates). Therefore, GSL clusters on cell surface may be the site involved in not only cell adhesion but also in initiation of signal transduction (Fig. 2). Although there is no evidence to support the hyposesis that GSL-GSL interaction occurs in vivo, it is possible that GSL-GSL interaction may play a role for recognition and/ or signaling events in a variety of biological phenomena.
| |
|
| Naoya Kojima (Tokai University, School of Engineering) | |
|
|
| References | (1) | Eggens, I, Fenderson, B, Toyokuni, T, Dean, B, Stroud, M, Hakomori, S J. Biol. Chem. 264, 9476-9484,1989 |
| (2) | Kojima, N, Hakomori, S J. Biol. Chem. 266, 17552-17558,1991 |
| (3) | Kojima, N, Shiota, M, Sadahira, Y, Handa, K, Hakomori, S J. Biol. Chem. 267, 17264-17270,1992 |
| (4) | Iwabuchi, K, Yamamura, S, Prinetti, A, Handa, K, Hakomori, S J. Biol. Chem. 273, 9130-9138,1998 |
| | |
| |
| Sep.15, 1998 |
|
| |
|
|
|
|



