 |
Binding of pathogenic bacteria and bacterial toxins to host cell surfaces is an essential step in establishing infection in tissues and producing toxic effect. Glycosphingolipids on cell surfaces are receptors for binding to cells.
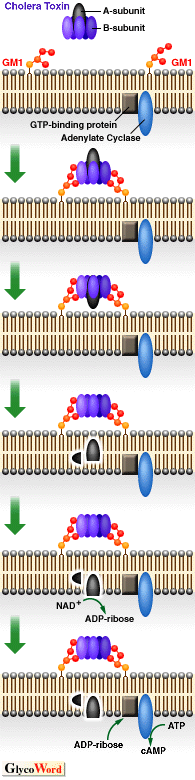
There are many bacterial toxins that bind to ganglioside, an acid glycosphingolipid, as the receptor on cell surface and invade host cells. The best known of these is the cholera toxin, an enterotoxin produced by Vibrio cholerae, and its specific cell surface receptor was identified as ganglioside GM1. Cholera toxin consists of a pentameric B subunit that binds to GM1 and an A subunit with direct toxic activity. The binding of B subunit to membrane GM1 may induce a conformational change in the toxin, resulting in the entry of the A subunit into the cell. The B subunit contains 103 amino acid residues, and Arg-35 and Trp-88 participate in binding to GM1. The mechanism of binding the heat-labile toxin produced by enterotoxic Escherichia coli, which is structurally related to cholera toxin is similar to the mechanism of cholera toxin binding. Other ganglioside-binding bacterial toxins include Tetanus toxin (GD1b), botulinum toxins (GT1b and GQ1b) and delta toxin produced by Clostridium perfringens (GM2). Shiga toxin produced by Shigella dysenteriae and Vero toxin produced by enterohaemorrhagic E. coli bind to neutral glycosphingolipids having an alpha-1,4 galabiose moiety in the sugar chain, such as galabioside (Ga2Cer) and ceramide trihexoside (Gb3Cer).
While many pathogenic bacteria also bind to glycosphingolipids of host cell surface for colonization and infection, uropathogenic E. coli which cause urinary tract infections can bind to glycosphingolipids having an alpha-1,4 galabiose moiety at the non-reducing end of the sugar chain (Gb3Cer, etc). There is evidence that bacteria bind to glycosphingolipid receptor by recognizing not only the terminal sequence but also the internal sequence of the sugar chain. Uropathogenic E. coli can also bind to globoside (Gb4Cer) and Forssman glycolipid, both of which have an alpha-1,4 galabiose moiety internally in a sugar chain. Such receptor substances are designated as isoreceptors. E. coli binds to glycosphingolipids by pili which exist on the bacterial cell surface and are similar to fibers or hairs. On the top of pili, there is an adhesin characterized as a lectin. Several types of adhesin, with respect to their sugar specificity, have been identified. They are the type I adhesin of E. coli that are mannose specific, type P adhesin, also of E. coli, specific for alpha-1,4 galabiose moiety, and type S adhesin of E. coli, specific for sialylgalactose moiety. It was reported that the amino acid sequence of P-adhesin is similar as that of Shiga toxin because both recognize alpha-1,4 galabiose moiety of glycosphingolipid. Propionibacterium, which causes skin disease, recognizes the lactosyl moiety of glycosphingolipids as a binding epitope. These bacteria can bind strongly to lactosylceramide and also bind to isoreceptors such as asialo GM1 (GA1) and asialo GM2 (GA2). Because almost all glycosphingolipids contain a common lactosyl moiety, Propionibacterium may be assumed to bind almost all glycosphingolipids. However, the bacteria cannot bind to any glycosphingolipids composed of a dihydroxy base and nonhydroxy fatty acid in ceramide, even though these contain a lactosyl moiety. This fact indicates that the binding epitope of the bacteria also depends on the ceramide structure in addition to the lactosyl moiety in sugar chain. Neisseria gonorrhoeae, which cause gonorrhoea, also bind glycosphingolipids having a lactosyl moiety.
Conformational analysis of glycosphingolipids with terminal and internal alpha-1,4 galabiose moiety showed that conformation at this disaccharide is almost identical for all glycosphingolipids with a bend in the sugar chain. The convex side of the bend probably carries the binding epitope for many batceria.
Recently, it was found that Lactobacillus, a representative of useful bacteria found in the intestinal tract, specifically binds to some glycosphingolipids. These bacteria can bind to neutral glycosphingolipids but not to gangliosides.
|  |  |
|
|
| References | (1) | Karlsson, K-A : Animal glycosphingolipids as membrane attachment sites for bacteria. Annu.Rev.Biochem. 58, 309-350, 1989 |
| (2) | Bock,K, Breimer, ME, Brignole, A, Hansson, GC, Karlsson, K-A, Larson, G, Leffler, H, Samuelsson, BE, Stromberg, N, Eden, CS : Specificity of binding of a strain of uropathogenic Escherichia coli to Gal alpha 1-4 Gal-containing glycosphingolipids. J.Biol.Chem. 260, 8545-8551, 1985 |
| (3) | Holmgren, J : Actions of cholera toxin and the prevention and treatment of cholera. Nature, 292, 413-417, 1981 |
| | |
| |



