| Localization of Glycosphingolipids by an Immunochemical Technique |  |
|
 |
A large number of molecular species of glycosphingolipids have been identified based on the diversity of their carbohydrate structures. They are mainly expressed on the outer surface of plasma membranes in the vertebral animals. They play essential roles in a variety of phenomena involving cell-cell recognition, cell adhesion, signal transduction, and cell growth and differentiation. Also, it is known that glycolipids are the receptors of some bacteria and viruses. Glycolipids have been identified as tumor or differentiation antigens using monoclonal antibodies (MAbs) generated by a hybridoma technology. To date, however, the localization of glycolipids in cells and tissues has not been characterized well because of the lack of useful probes for recognizing them specifically.
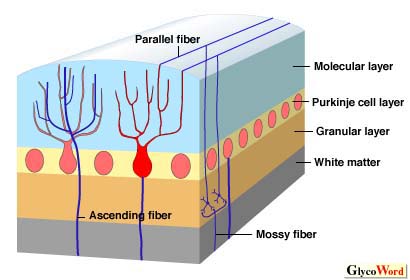
An improved method for the generation of MAbs specific for carbohydrate-chains was recently established by immunizing mice with purified glycolipids or neo-glycolipids. Using this method, a number of sets of MAbs specific for ganglio-, gala-, globo-series glycolipids, and for the carbohydrates of N-linked glycoproteins have been generated and their binding specificities characterized. There are a number of applications in the field of medicine and life science. These MAbs enabled us to examine the distribution of gangliosides (sialic acid-containing glycosphingolipids) in the adult rat brain. The study revealed the differential distribution patterns of gangliosides in the cell layers of the brain regions. For example, in cerebellar cortex, GM1 was expressed in the white matter. GD1a was detected exclusively in the molecular layer. GD1b and GQ1b were present restrictedly on the granular layer; GD1b was detected on the surface of the granular cell bodies, whereas GQ1b was present in the cerebellar glomerulus. GT1b was distributed densely in the whole layers except the Purkinje cell layer. (Table 1) Also, it was found that the expression of each ganglioside changes dramatically during the development of postnatal rat cerebellar cortex by the same technique. Furthermore, a study revealed that there is a cell type-specific expression of gangliosides in the rat cerebellar primary cultures. GD1b and O-Ac-LD1 were restrictedly detected in certain types of small neurons and large neurons, respectively. Gb4Cer was detected in astrocytes, whereas galactosylceramide and sulfatide were distributed specifically in oligodendrocytes. These results suggest that several glycolipids may be useful markers for identifying cells in primary cultures of the rat cerebellum. A differential distribution was also found in the cell layers of adult rat small intestine.
| |
|
| Figure1 The structure of cerebellar cortex
 |
|
|
|
|
|
It was previously reported that the composition of gangliosides shows little difference in the brain regions using a biochemical technique. In general, however, it has been widely accepted that a number of significant differences are observed in ganglioside composition quantitatively and qualitatively between fetal and adult brains, whereas no significant differences are found among cells or cell layers in the central nervous system. Some caution must be exercised in interpreting glycolipid localization based on immunohistochemistry, since a lack of immunorecognition of glycolipid epitope on cells and tissues does not necessarily mean the absence of glycolipids. It is somewhat easy to eliminate the possibility of non-specific binding when a glycolipid antigen detection is positive. There are indications that a number of factors are involved in influencing the reactivity of MAbs with specific cells and tissues. Further study will be needed for elucidating the precise mechanisms of immunoreactivity. An immunoelectron microscopic study will be necessary to further evaluate the localization of the glycolipids in cells and tissues.
Thus, immunohisto- and immunocyto-chemical studies with a series of Mabs specific for glycolipids suggest that there is a differential distribution of glycolipids in a variety of cells and tissues. These results are expected to be an important basis for elucidating their physiological and pathological roles in cells and tissues in the near future.
| |
|
| Tadashi Tai (The Tokyo Metropolitan Institute of Medical Science, Tumor Immunology) | |
|
|
| References | (1) | Kotani, M, Terashima, T, Tai, T : Developmental changes of ganglioside expressions in postnatal rat cerebellar cortex. Brain Res. 700, 40-58, 1995 |
| (2) | Kawashima, I, Nagata, I, Tai, T : Immunocytochemical analysis of gangliosides in rat primary cerebellar cultures using specific monoclonal antibodies. Brain Res. 732, 75-86, 1996 |
| (3) | Tai, T, Kawashima, I, Ozawa, H, Kotani, M, Ogura, K : Cell type-specific expression of ganglioside antigens in the central nervous system. Pure and Appl. Chem, 69. 1903-1910, 1999 |
| | |
| |
|
| Jun.15, 1998 |
|
| |
|
|
|
|






