|
N-Glycolylneuraminic Acid and N-Acetylneuraminic Acid
|
 |
|
 |
Guntar Blix isolated an acidic aminosugar from sialomucin by acid hydrolysis in a crystallized form and named it sialic acid. Ernst Klenk also isolated a similar crystallized aminosugar from brain gangliosides by acid hydrolysis and named it neuraminic acid. Both acidic aminosugars were identified to be the same molecule and the correct structure was finally proposed by Alfred Gottshalk. Over 30 derivatives of neuraminic acids, N-acetylneuraminic acid (NeuAc) and N-glycolylneuraminic acid (NeuGc) as the core structures, are found in natural materials isolated from living organisms. Guntar Blix, Ernst Klenk, and Alfred Gottshalk reached the agreement that the term sialic acid be used as the family name covering all derivatives of neuramininic acid.
Patients treated with sera of horse, sheep, or goat develop antibodies against heterophile or xenogenic antigens of these animals. Naiki and colleagues in Japan and Milgrom and colleagues in the U.S. found one of the xenogenic antigens to be NeuGc containing glycoconjugates. No report has been published presenting the chemical evidence for the occurrence of NeuGc in glycolipids and glycoproteins of normal human tissues. Therefore, our understanding is that NeuGc is recognized as a foreign material by the immune system and that anti-NeuGc antibody is produced by the injection of NeuGc containing glycoconjugates which are quite rich in the sera of these animals. |
|
|
|
|
|
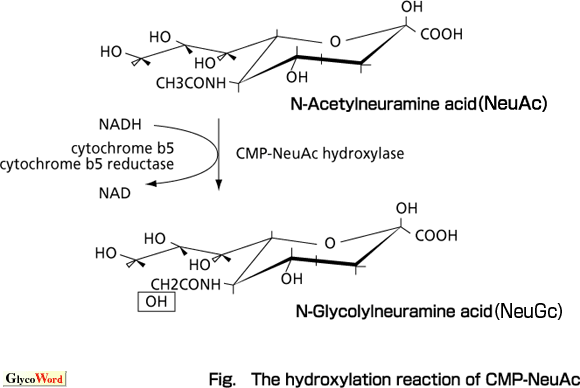
Schauer and colleagues have conducted an extensive investigation on the structures, biosynthesis and functions of the sialic acid family, noting for the first time that NeuGc is biosynthesized from NeuAc and the rate limiting step responsible for NeuGc expression is the hydroxylation reaction of CMP-NeuAc. Kozutsumi in our group found that the hydroxylation of CMP-NeuAc requires cytochrome b5, and on the basis of this finding we were able to demonstrate that the hydroxylation reaction is a complex reaction carried out by NADH, NADH-cytochrome b5 reductase, cytochrome b5, and the terminal enzyme hydroxylase. Then the hydroxylase was purified from mouse liver cytosol and its cDNA cloned. Northern blotting with mouse cDNA as a probe proved that mouse organs except the brain express hydroxylase mRNA. This suppression in the brain is conserved even in humans and we believe that the presence of NeuGc in brain glycoconjugates may not be favorable for brain functions, so there must be a suppression mechanism specific in the brain.
Unexpectedly we found that humans have a sequence homologous to mouse cDNA in the genome then cloned a cDNA from a HeLa cell library. The sequence indicated that human cDNA lacks a 92-bp fragment which corresponds to mouse exon 5, and a transfection experiment proved that this cDNA does not produce enzyme activity. We could not find any parts of the 92-bp sequence in the human genomic sequence corresponding to mouse introns 4 and 5. Ajit Varki recently demonstrated that this 92- bp deletion is not found in the cDNA of chimpanzee hydroxylase, indicating that the deletion happened after a human ancestor had diverged from a chimpanzee ancestor.
What changes were produced by the lack of NeuGc expression in humans? Even today, infection threatens our lives. E. coli K99 is known to carry a molecule which recognizes NeuGc containing GM3 and produces severe diarrhea in newborn pigs. Human babies can escape from this threat. We are also immune to influenza virus carrying hemagglutinin specific to NeuGc. The suppression of CMP-NeuAc hydroxylase mRNA in the brain seems to be conserved among mammals, suggesting an important physiological role for NeuAc in the brain. The loss of NeuGc expression results in the loss of expression of more than several tens of carbohydrate chains of glycoproteins and glycolipids and we do not know what this means in terms of survival of humans in the past or the future. |
|
|
|
Akemi Suzuki (Tokyo Metropolitan Institute of Medical Science) |
|
|
|
|
|
| References |
(1) |
T. Kawano, S. Koyama, H. Takematsu, Y. Kozutsumi, H. Kawasaki, S. Kawashima, T.Kawasaki, A. Suzuki : Molecular cloning of cytidine monophospho-N-acetylneuraminic acid hydroxylase. Regulation of species- and tissue-specific expression of N-glycolylneuraminic acid. J. Biol. Chem. 270, 16458-16463, 1995 |
|
(2) |
A. Irie, S. Koyama, Y. Kozutsumi, T. Kawasaki, A. Suzuki : The Molecular basis for the absence of N-glycolylneuraminic acid in humans. J. Biol. Chem. 273, 15866-15871, 1998 |
|
(3) |
H.-H. Chou, H. Takematsu, S. Diaz, J. Iber, E. Nickerson, K L. Wright, E A. Muchmore, D L. Nelson, S T. Warren, A. Varki : A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc. Natl. Acad. Sci. USA, 95, 1171-11756, 1998 |
|
|
|
|
|
| Jun. 15, 1999 |
|
|
|
|
|
|



