 |
Syndecan-4 is a transmembrane heparan sulfate proteoglycan belonging to the syndecan family. The core protein has a molecular weight of about 30 kDa. In the extracellular domain, there are three attachment sites of glycosaminoglycan chains. Syndecan-4 is found as an antihrombin binding molecule, and is also called ryudocan or amphiglycan.
Heparan sulfate chains of syndecan-4 are considered to play various roles by binding to growth factors such as basic fibroblast growth factor (bFGF) and midkine, anticoagulation factors such as tissue factor pathway inhibitor, and cell adhesion molecules such as fibronectin. In many cases, the signal of ligand binding to heparan sulfate is transmitted to the intracellular signaling system or to the cytoskeleton via the cytoplasmic domain of the core protein. We produced knockout mice deficient in syndecan-4 and analyzed its function.
First, we examined the involvement of syndecan-4 in focal adhesion formation. Focal adhesion is a molecular complex formed at the site of cell adhesion to substratum, from which bundles of actin fibers extend. Focal adhesion is believed to play important roles also in vivo. Formation of focal adhesion requires the cooperation of two signals from two domains of fibronectins, namely, the cell-binding domain, which binds to integrin, and the heparin-binding domain, which binds to heparan sulfate proteoglycans. One of the latter has been identified as syndecan-4. We examined whether the syndecan-4-deficient [Synd4(-/-)] fibroblasts from Synd4(-/-) mice produce focal adhesion. To our surprise, focal adhesion was formed in the deficient cells, indicating that heparan sulfate proteoglycans other than syndecan-4 compensated for the loss of syndecan-4. However, when the heparin-binding fragment was given only from the liquid surface, the wild type [Synd4(+/+)] cells formed focal adhesion but the deficient cells did not. In vivo, an analogous situation is expected to occur upon inflammation.
When the distribution of syndecan-4 in blood vessels is examined, fetal vessels in the placenta expressed only syndecan-4 among syndecan family members. Thus, we compared thrombi formation between Synd4(+/+) and Synd4(-/-) mice and found that it was significantly more frequent in Synd4(-/-) mice. The result confirmed that heparan sulfate chains on syndecan-4 play roles in endogeneous anticoagulation mechanisms.
 -Carrageenan induces obstructive nephritis upon injection into mice; it is deposited in the renal collecting tubules. -Carrageenan induces obstructive nephritis upon injection into mice; it is deposited in the renal collecting tubules.  -Carrageenen-induced nephitis was more severe in Synd4(-/-) mice than in Synd4(+/+) mice. Syndecan-4 is considered to bind to basic substances in the renal collecting tubules, thereby preventing the deposition of toxic substances. -Carrageenen-induced nephitis was more severe in Synd4(-/-) mice than in Synd4(+/+) mice. Syndecan-4 is considered to bind to basic substances in the renal collecting tubules, thereby preventing the deposition of toxic substances.
Septic shock upon bacterial infection is a serious cause of death in intensive care units. Septic shock caused by endotoxin is frequently used as an experimental model of septic shock. Synd4 (-/-) mice were more susceptible to endotoxin shock than Synd4 (+/+) mice, and the mortality upon the shock was also higher in Synd4(-/-) mice.
The principal cause of endotoxin shock was the excessive production of inflammatory cytokines such as IL-1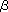 or TNF or TNF . It is also known that TGF- . It is also known that TGF-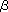 1 is involved in the feedback machanism to suppress the production of inflammatory cytokines. We found that the feedback mechanism was impaired in Synd4(-/-) mice, that TGF- 1 is involved in the feedback machanism to suppress the production of inflammatory cytokines. We found that the feedback mechanism was impaired in Synd4(-/-) mice, that TGF-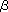 1 bound to syndecan-4 and that syndecan-4 expression in macrophages and in blood vessels increased upon endotoxin administration. Therefore, syndecan-4 is considered to bind to TGF- 1 bound to syndecan-4 and that syndecan-4 expression in macrophages and in blood vessels increased upon endotoxin administration. Therefore, syndecan-4 is considered to bind to TGF-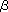 1 and participate in the feedback mechanism by augmenting the action of TGF- 1 and participate in the feedback mechanism by augmenting the action of TGF-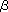 1. 1.
Synd4(-/-) mice was also reported to be impaired in the repair of injured skin. In conclusion, all functions of syndecan-4 so far clarified are related to the defense mechanism against either injury or bacterial infection. |
|
|
| References |
(1) |
Kojima T, Shworak NW, Rosenberg RD :Molecular cloning and expression of two distinct cDNA-encoding heparan sulfate
proteoglycan core proteins from a rat endothelial cell line, J Biol Chem 267, 4870-4877, 1992 |
|
(2) |
Kojima T, Katsumi A, Yamazaki T, Muramatsu T, Nagasaka T, Ohsumi K, Saito H : Human ryudocan from endothelium-like cells binds basic fibroblast growth factor, midkine, and tissue factor pathway inhibitor, J Biol Chem 271, 5914-5920, 1996 |
|
(3) |
Ishiguro K, Kadomatsu K, Kojima T, Muramatsu H, Tsuzuki S, Nakamura E, Kusugami K, Saito H, Muramatsu T : Syndecan-4 deficiency impairs focal adhesion formation only under restricted conditions, J Biol Chem 275, 5249-5252, 2000. |
|
(4) |
Ishiguro K, Kadomatsu K, Kojima T, Muramatsu H, Iwase M, Yoshikai Y, Yanada, M, Yamamoto K, Matsushita T, Nishimura M, Kusugami, K, Saito H, Muramatsu T : Syndecan-4 deficiency leads to high mortality of lipopolysaccharide- injected mice, J Biol Chem 276, 47483-47488, 2001 |
|
|
|
|
|



