|
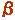 -1,4-Galactosyltransferase ( -1,4-Galactosyltransferase (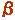 -1,4-GalT) Gene KO Mice -1,4-GalT) Gene KO Mice
|
 |
|
 |
 -1,4-Galactosyltransferase ( -1,4-Galactosyltransferase ( -1,4-GalT) (EC 2.4.1.38) is a glycosyltransferase that is required for the biosynthesis of the backbone structure from type 2 chain (Gal -1,4-GalT) (EC 2.4.1.38) is a glycosyltransferase that is required for the biosynthesis of the backbone structure from type 2 chain (Gal 1 1 4GlcNAc), which appears widely on N-glcans, O-glycans and glycolipids. The type 2 chain is particularly important in the synthesis of sialyl lewis x and SSEA-1, which play a role in the immune system and early embryogenesis, respectively. 4GlcNAc), which appears widely on N-glcans, O-glycans and glycolipids. The type 2 chain is particularly important in the synthesis of sialyl lewis x and SSEA-1, which play a role in the immune system and early embryogenesis, respectively.
Although  -1,4-GalT gene was believed to be a single gene in the past, novel -1,4-GalT gene was believed to be a single gene in the past, novel  -1,4-GalT genes were successively isolated in 1997-1998 to form the -1,4-GalT genes were successively isolated in 1997-1998 to form the  -1,4-GalT gene family consisting of seven genes. KO mice deficient in the -1,4-GalT gene family consisting of seven genes. KO mice deficient in the  -1,4-GalT-I gene, which was isolated for the first time, were generated by our group and Dr. Shur’s group (1, 2). A few residual -1,4-GalT-I gene, which was isolated for the first time, were generated by our group and Dr. Shur’s group (1, 2). A few residual  -1,4-GalT activity and faint bands corresponding to the Gal -1,4-GalT activity and faint bands corresponding to the Gal 1 1 4GlcNAc structure detected in 4GlcNAc structure detected in  -1,4-GalT-I KO mice could be derived from other -1,4-GalT-I KO mice could be derived from other  -1,4-GalT gene(s). -1,4-GalT gene(s). |
|
|
 |
|
|
|
|
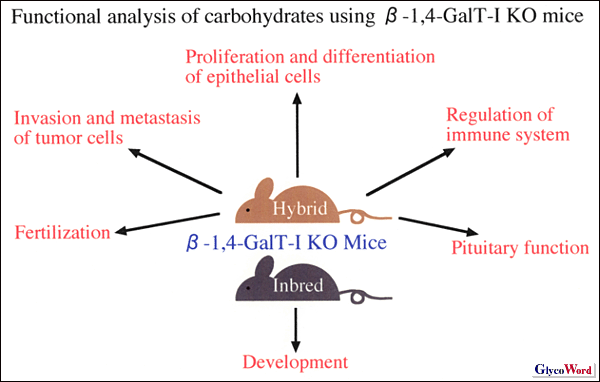
 -1,4-GalT-I KO mice were unexpectedly born normally, although an essential role of the Gal -1,4-GalT-I KO mice were unexpectedly born normally, although an essential role of the Gal 1 1 4GlcNAc structure was suggested in early embryogenesis. However, they showed growth retardation after birth and half of them died before the weaning period. Dr. Shur’s group suggested that the neonatal lethality was caused by pituitary insufficiency due to agalactosylation of pituitary hormones (2). On the other hand, our data suggest that abnormal differentiation of intestinal villus cells, especially reduced expression level of lactase, caused the growth retardation and semi-lethality before weaning (1). 4GlcNAc structure was suggested in early embryogenesis. However, they showed growth retardation after birth and half of them died before the weaning period. Dr. Shur’s group suggested that the neonatal lethality was caused by pituitary insufficiency due to agalactosylation of pituitary hormones (2). On the other hand, our data suggest that abnormal differentiation of intestinal villus cells, especially reduced expression level of lactase, caused the growth retardation and semi-lethality before weaning (1).  -1,4-GalT-I KO mice showed augmented proliferation and abnormal differentiation of intestinal and epidermal epithelial cells, suggesting the carbohydratechains synthesized by -1,4-GalT-I KO mice showed augmented proliferation and abnormal differentiation of intestinal and epidermal epithelial cells, suggesting the carbohydratechains synthesized by  -1,4-GalT-I regulate proliferation and differentiation of epithelial cells (1). -1,4-GalT-I regulate proliferation and differentiation of epithelial cells (1).
 -1,4-GalT-I was suggested to be involved in fertilization in a past study of Dr. Shur’s group since the sperm surface-localized long isoform of -1,4-GalT-I was suggested to be involved in fertilization in a past study of Dr. Shur’s group since the sperm surface-localized long isoform of -1,4-GalT-I interacted with ZP3 on eggs. However, -1,4-GalT-I interacted with ZP3 on eggs. However,  -1,4-GalT-I KO mice of both sexes were fertile in natural mating. Shur’s group showed that sperm from -1,4-GalT-I KO mice of both sexes were fertile in natural mating. Shur’s group showed that sperm from  -1,4-GalT-I KO mice bound with ZP3 and penetrated the egg coat less effectively compared to wild-type mice during in vitro fertilization, suggesting other binding molecules between sperm and eggs could compensate for the -1,4-GalT-I KO mice bound with ZP3 and penetrated the egg coat less effectively compared to wild-type mice during in vitro fertilization, suggesting other binding molecules between sperm and eggs could compensate for the  -1,4-GalT-I deficiency in natural mating(3). -1,4-GalT-I deficiency in natural mating(3).
To understand the effects of the  -1,4-GalT-I deficiency on carbohydrate structures, N-glycans and O-glycans in various tissues of -1,4-GalT-I deficiency on carbohydrate structures, N-glycans and O-glycans in various tissues of -1,4-GalT-I KO mice were examined. Although ploysialic acid and HNK-1 carbohydrate expressed on Gal -1,4-GalT-I KO mice were examined. Although ploysialic acid and HNK-1 carbohydrate expressed on Gal  4GlcNAc outer chains play an important role in neural structure and function, 4GlcNAc outer chains play an important role in neural structure and function,  -1,4-GalT-I KO mice showed normal behavior with normal expression levels of these carbohydrates in the brain(4). Residual -1,4-GalT-I KO mice showed normal behavior with normal expression levels of these carbohydrates in the brain(4). Residual  -1,4-GalT-I activity in the mutant brain was as high as 65% in the wild-type brain, suggesting that other -1,4-GalT-I activity in the mutant brain was as high as 65% in the wild-type brain, suggesting that other  -1,4-GalT gene(s) worked in the brain. -1,4-GalT gene(s) worked in the brain.
Analysis of the carbohydrate structure of erythrocyte membrane glycoproteins of  -1,4-GalT-I KO mice revealed that -1,4-GalT-I KO mice revealed that  -1,4-GalT-I was mainly responsible for -1,4-GalT-I was mainly responsible for -1,4-galactosylation of the core 2 O-glycan branch in erythrocytes(5). -1,4-galactosylation of the core 2 O-glycan branch in erythrocytes(5).
Moreover, detailed examination of N-glycans of hepatic membrane glycoproteins and plasma glycoproteins disclosed that not only was the synthesis of the backbone structure from the type 2 chain (Gal 1 1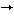 4GlcNAc) reduced, but that the synthesis of the backbone structure from the type 1 chain (Gal 4GlcNAc) reduced, but that the synthesis of the backbone structure from the type 1 chain (Gal 1 1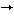 3GlcNAc) was enhanced. 3GlcNAc) was enhanced.
Furthermore, the change in the backbone structure in N-glycans shifted sialyl linkage from Sia 2 2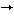 6Gal to Sia 6Gal to Sia 2 2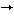 3Gal. These results indicate that the 3Gal. These results indicate that the -1,4-GalT-I deficiency does not simply result in a loss of the type 2 chain, but causes marked alteration of the backbone structure (6). -1,4-GalT-I deficiency does not simply result in a loss of the type 2 chain, but causes marked alteration of the backbone structure (6).
These results must be taken into careful consideration when phenotypes of  -1,4-GalT-I KO mice are interpreted. -1,4-GalT-I KO mice are interpreted.
Recent analysis of  -1,4-GalT-I mice revealed that the synthesis of the core 2 O-glycan branch was also reduced in mutant leukocytes and binding of selectins to mutant leukocytes was reduced, resulting in impaired inflammatory responses. -1,4-GalT-I mice revealed that the synthesis of the core 2 O-glycan branch was also reduced in mutant leukocytes and binding of selectins to mutant leukocytes was reduced, resulting in impaired inflammatory responses.  -1,4-GalT-I may play an important role in development because inbred -1,4-GalT-I may play an important role in development because inbred  -1,4-GalT-I KO mice showed embryonic lethality. -1,4-GalT-I KO mice showed embryonic lethality.
Very recently, a patient with a mutation in the  -1,4-GalT-I gene was identified and his syndrome named as CDG-IId (7). A truncated -1,4-GalT-I gene was identified and his syndrome named as CDG-IId (7). A truncated  -1,4-GalT-I protein lacking 50 amino acids of the C-terminus was expressed but did not localize in the Golgi apparatus. Residual -1,4-GalT-I protein lacking 50 amino acids of the C-terminus was expressed but did not localize in the Golgi apparatus. Residual  -1,4-GalT activity (5% of that in normal serum) in the patient serum was as much as that in the serum of -1,4-GalT activity (5% of that in normal serum) in the patient serum was as much as that in the serum of -1,4-GalT-I KO mice, suggesting a null mutation in human. -1,4-GalT-I KO mice, suggesting a null mutation in human.
However, the patient showed hydrocephalus, myopathy and blood-clotting defects, inconsistent with  -1,4-GalT-I KO mice. As there is only one example in human, detailed comparison between mouse and human is required. -1,4-GalT-I KO mice. As there is only one example in human, detailed comparison between mouse and human is required. |
|
|
|
Masahide Asano
(Institute for Experimental Animals, Graduate School of Medical Science,
Kanazawa University) |
|
|
|
|
|
| References |
(1) |
Asano M, et al.: Growth retardation and early death of  -1,4-galactosyltransferase knockout mice with augmented proliferation and abnormal differentiation of epithelial cells. EMBO J. 16,1850-1857, 1997 -1,4-galactosyltransferase knockout mice with augmented proliferation and abnormal differentiation of epithelial cells. EMBO J. 16,1850-1857, 1997 |
|
(2) |
Lu Q, Hasty P, Shur BD,: Targeted mutation in  -1,4-galactosyltransferase leads to pituitary insufficiency and neonatal lethality. Dev. Biol. 181, 257-267, 1997 -1,4-galactosyltransferase leads to pituitary insufficiency and neonatal lethality. Dev. Biol. 181, 257-267, 1997 |
|
(3) |
Lu Q, Shur, BD,: Sperm from  1,4-galactosyltransferase-null mice are refractory to ZP3-induced acrosome reactions and penetrate the zona pellucida poorly. Development 124, 4121-4131, 1997 1,4-galactosyltransferase-null mice are refractory to ZP3-induced acrosome reactions and penetrate the zona pellucida poorly. Development 124, 4121-4131, 1997 |
|
(4) |
Kido M, et al.: Presence of polysialic acid and HNK-1 carbohydrate on brain glycoproteins from  -1,4-galactosyltransferase-knockout mice. Biochem. Biophys. Res. Commun. 245, 860-864, 1998 -1,4-galactosyltransferase-knockout mice. Biochem. Biophys. Res. Commun. 245, 860-864, 1998 |
|
(5) |
Kotani N, et al.: Impaired  -1,4-galactosylation of core 2 0-glycans in erythrocytes of -1,4-galactosylation of core 2 0-glycans in erythrocytes of  -1,4-galactosyltranferase knockout mice. Biochem. Biophys. Res.Commun. 260, 94-98, 1999 -1,4-galactosyltranferase knockout mice. Biochem. Biophys. Res.Commun. 260, 94-98, 1999 |
|
(6) |
Kotani N, et al.: Knockout of mouse  1,4-galactosyltransferase-1 gene results in a dramatic shift of outer chain moieties of N-glycans from type 2 to type 1 chains in hepatic membrane and plasma glycoproteins. Biochem. J. 357, 827-834, 2001 1,4-galactosyltransferase-1 gene results in a dramatic shift of outer chain moieties of N-glycans from type 2 to type 1 chains in hepatic membrane and plasma glycoproteins. Biochem. J. 357, 827-834, 2001 |
|
(7) |
Hansske B, et al.: Deficiency of UDP-galactose: N-acetylglucosamine  -1,4-galactosyltransferase-I causes the congenital disorder of glycosylation type IId. J. Clin. Invest. 109, 725-733, 2002 -1,4-galactosyltransferase-I causes the congenital disorder of glycosylation type IId. J. Clin. Invest. 109, 725-733, 2002 |
|
|
|
|
|
| Jun. 15, 2002 |
|
|
|
|
|
|



