 |
Notch receptors mediate an evolutionarily conserved intercellular signaling that regulates a wide range of cell fate decision processes. For precise control of Notch activity during development, various modulators act at different levels of the signaling pathway. One that directly modifies on Notch activity is sugar modification such as O-fucose glycan. O-fucose glycan is initially found as a rare type of post-translational modification on EGF domain-containing serum proteins including blood clotting Factors VII and IX. Moreover, Notch receptors, membrane proteins that contain approxi-mately 36 tandemly arrayed EGF domains, are shown to be O-fucosylated. The consensus sequence (C2XXX(A/G/S)(S/T)C3) predicts that more than 20 EGF domains can be modified with O-fucose. Notch ligands, Delta and Serrate/Jagged, are also reported to be O-fucosylated.
The common structure of O-fucose glycan on the EGF domain is Fucose-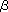 1,3-GlcNAc- 1,3-GlcNAc-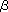 1,4-Gal-a2,3/2,6-Sialic acid. Glycosylation process is mediated by the sequential action of enzymes called glycosyltransferases. For synthesizing the unique structure of O-fucose glycans, specific glycosyl-transferases are employed. One of these is a polypeptide O-fucosyltransferase1 (OFUT1) which first acts in the glycosylation pathway and transfers fucose onto Ser/Thr residue within the consensus sequence (Figure, (A)) (therefore called O-linked fucose or O-fucose). This O-fucose monosaccharide is further elongated by an O-fucose specific GlcNAc transferase, Fringe. OFUT1 and Fringe are shown to function in distinct compartments in the secretion pathway. OFUT1 is a soluble endoplas-mic reticulum (ER) protein that carries a KDEL sequence at the carboxyl terminus, which serves as a retrograde transport from cis-Golgi to ER. Although Fringe is originally known as secreted molecules, it is reported to localize in Golgi. The expression pattern of fringe is spatially and temporarily regu-lated, whereas Ofut1 expression is largely ubiquitous. 1,4-Gal-a2,3/2,6-Sialic acid. Glycosylation process is mediated by the sequential action of enzymes called glycosyltransferases. For synthesizing the unique structure of O-fucose glycans, specific glycosyl-transferases are employed. One of these is a polypeptide O-fucosyltransferase1 (OFUT1) which first acts in the glycosylation pathway and transfers fucose onto Ser/Thr residue within the consensus sequence (Figure, (A)) (therefore called O-linked fucose or O-fucose). This O-fucose monosaccharide is further elongated by an O-fucose specific GlcNAc transferase, Fringe. OFUT1 and Fringe are shown to function in distinct compartments in the secretion pathway. OFUT1 is a soluble endoplas-mic reticulum (ER) protein that carries a KDEL sequence at the carboxyl terminus, which serves as a retrograde transport from cis-Golgi to ER. Although Fringe is originally known as secreted molecules, it is reported to localize in Golgi. The expression pattern of fringe is spatially and temporarily regu-lated, whereas Ofut1 expression is largely ubiquitous.
Using genetic techniques, the biological roles of Ofut1 and Fringe for Notch receptors are being addressed. In contrast, the roles in other substrates are largely unknown. Ofut1 is essential for Notch activation. As a molecular basis for this absolute requirement, it is reported that OFUT1 is required for proper conformation of Notch receptors suitable for ligand binding. Therefore, without Ofut1, Notch receptors are misfolded, which leads to loss of expression at the cell surface and ability to bind ligands. On the other hand, Fringe differentially modulates Delta and Serrate binding to Notch recep-tors; Fringe facilitates Notch-Delta binding whereas it inhibits Notch-Serrate binding. This Fringe effect is considered to be a central mechanism for local Notch activation at the Fringe expression boundary.
Despite great advances in understanding the roles of glycosyltransferases for Notch functions, the molecular mechanisms by which an individual O-glycan exerts regulatory functions still remains largely unknown. Based on the experiments of deletion mapping in Drosophila culture cells, it was shown that the 11th and 12th of EGF domains (EGF11 and EGF12) are the minimal binding units required for physical interaction with the ligands. Among them, only EGF12 carries the glycosylation site for O-fucose modification. Therefore, the simplest scenario is that an O-fucose glycan on EGF12 regulates the Notch-ligand interaction. However, the removal of the consensus sequence on EGF12 neither leads to loss of Notch activity nor Fringe effect. This implies that multiple O-fucose glycans cooperate to achieve glycosylation-dependent change of Notch-ligand binding. Alternatively, multiple O-fucose glycans may mediate the change in conformation of entire EGF repeats, thus indirectly affecting Notch-ligand interaction. On the other hand, OFUT1 was recently reported to possess chap-eron activity that is separable from the enzyme activity of OFUT1, a fact which may indicate that O-fucose modification is not essential for Notch function but rather modulates Notch activity like Fringe.
Comparison of the distribution of the O-fucose consensus sequence within EGF repeats of Notch among multiple species revealed that the glycosylation pattern is evolutionarily highly conserved. Therefore, it appears that the O-fucosylation sites on the EGF repeat are not distributed at random, but must be properly arranged. Careful analysis of glycosylation patterning on Notch will lead to the elucidation of molecular mechanisms for glycan-mediated regulation of Notch receptor functions.
|
|
|
| References |
(1) |
Haltiwanger RS, Stanley P: Modulation of receptor signaling by glycosylation: fringe is an O-fucose-beta1,3-N-acetylglucosaminyltransferase. Biochim Biophys Acta, 1573, 328-335, 2002 |
|
(2) |
Okajima T, Xu A, Lei L, Irvine KD: Chaperone activity of protein O-fucosyltransferase 1 promotes notch receptor folding. Science, 307, 1599-1603, 2005 |
|
(3) |
Okajima T, Xu A, Irvine KD: Modulation of notch-ligand binding by protein O-fucosyltransferase 1 and fringe. J Biol Chem, 278, 42340-42345, 2003 |
|
(4) |
Okajima T, Irvine KD: Regulation of notch signaling by o-linked fucose. Cell, 111, 893-904, 2002 |
|
|
|
|
|



