|
Differentiation of L-Type Lectin Domains into
Two Directions
|
|
|
 |
More than one hundred leguminous lectins have been isolated
from the seeds of leguminosae plants and are widely used as tools
for purification and identification of specific glycoproteins. cDNA cloning,
amino acid sequence analyses, and X-ray crystallographic analyses of these
lectins demonstrate that these proteins consist of a large family of leguminous
lectins based on structural molecular characteristics and also on the
function concerning self-defense against fungi and viruses by aggregating
these organisms (see GlycoWord LE-A03).
It was recently reported that another kind of protein having structural
homology with these plant leguminous lectins was also present in animal
cells (1), and these proteins were classified as L-type lectins. Cargo
receptors having an L-type lectin domain at a lumenal part of their molecules
transport newly synthesized glycoproteins from the ER through the Golgi
to the cell surfaces. Four members of these receptors are reported; ERGIC-53,
which has been identified as a marker of ER and Golgi intermediate compartment,
VIP36, which has been purified from detergent-insoluble membrane fraction,
and their homologues ERGL and VIPL, respectively. Although both amino
acid residues participating with sugar- and metal-binding and three-dimensional
structures of these proteins are highly conserved among plant leguminous
lectins and cargo receptors, several different characteristics have been
clarified between leguminous lectins and cargo receptors. In the case
of cargo receptors, the catch/release mechanism of sugars is essential
for vectorial transport of cargo proteins in the cells. |
|
|
 |
|
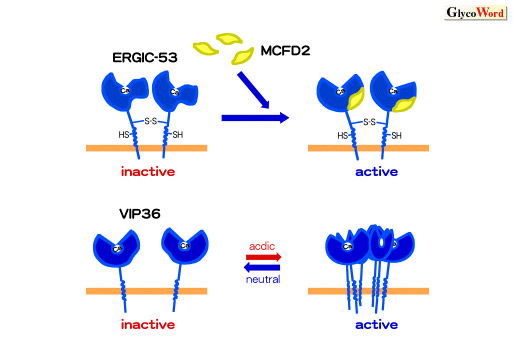
| Fig.1Regulation
of sugar-binding activities of cargo receptors, ERGIC-53 and VIP36
|
The sugar-binding activity of ERGIC-53 is enhanced
by binding with MCFD2 whereas the activity of VIP36 is increased
under acidic pH condition by the association of VIP36 with each
other.
|
|
|
|
|
|
ERGIC-53 can bind to sugars when it interacts with
MCFD2 (2). VIP36 associates with each other at acidic pH condition and
enhances avidity for sugar chains attached to cargo proteins (3) (Fig.
1). Regulation of sugar-binding activities of cargo receptors
is important for determining the direction of transport of cargo proteins.
In contrast, plant leguminous lectins have no release mechanism of ligands,
indicating that these lectins are suitable for aggregating fungi or
viruses to interfere their growth. L-type lectin domains have evolved
in two different directions and function as self-defense molecules in
plant seeds and transport receptors in the cells (Fig.
2).
|
|
|
 |
|
| Fig.2
Possible model for evolution of L-type lectin domains |
Plant leguminous lectins acquired the region which produces homotetramers stably. Cargo receptors have not only a stalk domain, transmembrane domain, and cytoplasmic domain but also a regulating region for sugar-binding activity in their molecules.
|
|
|
|
Kazuo Yamamoto
(Graduate School of Frontier Sciences, The University of Tokyo) |
|
|
|
|
|
| References |
(1) |
Fiedler K, Simons K: A putative novel class of animal lectins
in the secretory pathway homologous to leguminous lectins. Cell,
77, 625-626, 1994 |
|
(2) |
Zhang B, Cunningham MA, Nichols WC, Bernat JA, Seligsohn U, Pipe
SW, McVey JH, Schulte-Overberg U, de Bosch NB, Ruiz-Saez A, White
GC, Tuddenham EG, Kaufman RJ, Ginsburg D : Bleeding due to disruption
of a cargo-specific ER-to-Golgi transport complex. Nature Genet.
34, 220-225, 2003 |
|
(3) |
Yamamoto, K. Intracellular transport and sorting of glycoproteins
by cargo receptors. Seikagaku (in Japanese) 76,
pp240-255, 2004 |
|
|
|
| |
|
|
|
|
|
|
|
| Jun. 30, 2005 |
|
|
|
|
|
|
|






