

 |
 |
Comparative Biochemical Study on Glycosaminoglycans |
||||||||||||||||||||
 |
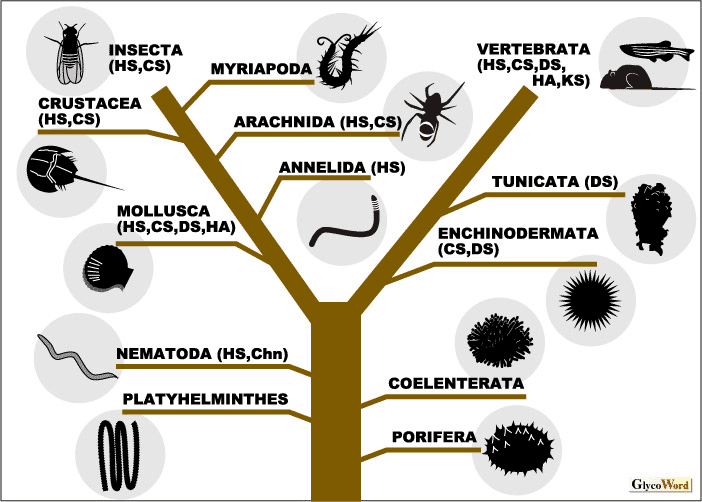
Sulfated glycosaminoglycans (GAGs) are widely distributed in the animal kingdom (Figure 1). Conversely, the presence of sulfated GAGs in the vegetable world has never been reported. Some bacterial strains synthesize polysaccharides with the same backbone structure as GAGs; however, these GAG-like polysaccharides are not attached to core proteins and are produced on the inner surface of the cytoplasmic membrane. Thus, the origin of these bacterial polysaccharides is most likely different from that of GAGs in metazoans. | |||||||||||||||||||
 |
||||||||||||||||||||
|
||||||||||||||||||||
|
A comparative study on the distribution of GAGs in full-scale classes of animals was undertaken by Dietrich et al. in the 1970s for the first time. They showed that sulfated polysaccharides assumed to be GAGs are distributed in not only vertebrates but also in invertebrates broadly ranging from Coelenterata to Insecta (1). However, it was difficult to quantitate GAGs or determine their disaccharide composition using the analytical techniques of that time, and the saccharide structure of invertebrate GAGs was not completely confirmed to be similar to that of vertebrate GAGs. Recently, sensitive microanalytical methods for sulfated GAGs using fluorescent tagging and HPLC have been established and applied to the quantitative analysis of GAG disaccharides at low picomole levels.
The nematode Caenorhabditis elegans is the lowest multicellular animal which has been so far demonstrated to produce GAGs using modern analytical techniques. It has been reported that C. elegans contains variously modified heparan sulfate (HS) and nonsulfated chondroitin chains (2). The fine structure of the chondroitin in C. elegans was determined by 1H NMR spectroscopy. C. elegans has been utilized as a model organism, and the fruit fly Drosophila melanogaster is also an ideal experimental organism. Biochemical studies on GAGs from Drosophila have been reported, and the structure of GAGs from Drosophila mutants lacking GAG biosynthetic enzymes has also been characterized (3). Drosophila produces diversely sulfated HS, as does C. elegans. However, chondroitin sulfate (CS) from Drosophila is lowly sulfated and composed of only nonsulfated and N-acetylgalactosamine-4-O-sulfated disaccharide units. Neither dermatan sulfate nor hyaluronan has been detected in these model organisms. The presence of dermatan sulfate has been reported in some invertebrates such as ascidians, sea urchins, and mollusks. From fish and amphibians to mammals, the structure of vertebrate GAGs is extremely heterogeneous. The biosynthesis of structurally complex GAG is regulated and its diverse sulfation pattern is formed organ- and tissue-specifically as well as temporally during growth and development. Mutational defects in most genes of GAG-biosynthetic enzymes in mice or zebrafish, which are representative model animals among the vertebrates, have been shown to lead to severe consequences, indicating that the expression of specific sulfation structures in GAGs plays an important role in life. When structural complexity in GAGs is compared with evolution, higher animals do not necessarily produce more highly sulfated GAGs. It has been shown that GAGs from sharks, hagfish, king crabs, squid, mollusks, sea cucumbers, and ascidians are characterized by oversulfated complex structures. There might be a tendency for aquatic species to contain more highly sulfated GAGs than terrestrial species. HS is widely distributed from very low organisms to humans, and diverse sulfation structures were revealed in HS from lower model animals such as C. elegans and Drosophila. However, reports on CS from lower organisms are limited. C. elegans synthesizes nonsulfated chondroitin but no CS chains. Therefore, HS might be involved in fundamental biological processes, whereas CS might have complex functions in higher organisms. Further studies will help to elucidate how life has acquired the huge structural complexity found in GAGs during evolution. |
||||||||||||||||||||
| Shuhei Yamada (Department of Biochemistry, Kobe Pharmaceutical University) | ||||||||||||||||||||
|
||||||||||||||||||||
| Jun. 30, 2006 | ||||||||||||||||||||
|
|
||||||||||||||||||||
|
||||||||||||||||||||