Core fucose in cancer | ||||||||||||||||||||||||
 |
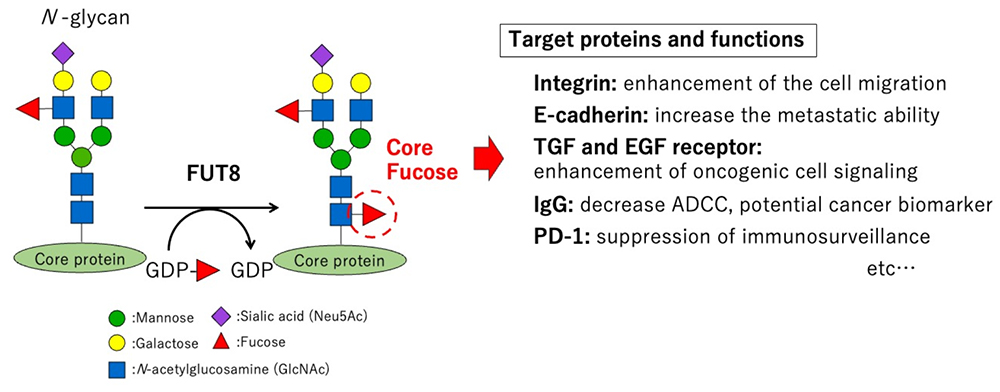
The core fucose structure of N-glycans is a fucose moiety linked to the innermost N-acetylglucosamine (GlcNAc) residue through an alpha 1,6 linkage. N-acetyl-β-d-glucosaminide α1,6 fucosyltransferase (FUT8) generates the core fucose structure in the Golgi apparatus by utilizing a sugar nucleotide, GDP-fucose, as a donor substrate. A total of 13 varieties of fucosyltransferases (FUTs) have been identified in human, but FUT8 is the only enzyme that generates the core fucose structure (1, 2). The roles of core fucose in cancer remain controversial. Most N-glycans can be modified by core fucose and cancer is a complicated phenomenon that accompanies many biological processes, making it extremely difficult to understand. The efforts of past and present eminent researchers, however, are gradually uncovering this mystery. For example, core fucose enhances the migration ability of cancer cells by increasing cell adhesion activity through integrin (3). Core fucose also regulates cell-cell adhesion by modifying the function of E-cadherin, which promotes metastasis (4). Focusing on growth factor receptors has shown that core fucosylation of transforming growth factor (TGF) receptors and epidermal growth factor (EGF) receptors strengthens the cell signaling that is transduced through these receptors, which results in faster growth of cancer cells (5, 6). The positive correlation between FUT8 expression level and tumor malignancy in some types of cancer has left little doubt that core fucose plays a crucial role in cancer (7). In our experiments, however, knocking out the FUT8 gene has not always suppressed the malignant phenotypes of cancer cells, which suggests that further investigations are needed to conclude. Antibody therapy is frequently selected in recent regimens for cancer treatment. As its name indicates, antibody therapy uses an antibody that targets the cell surface molecules expressed in cancer cells. The antibody is humanized and administrated in such a way as to induce the killing of cancer cells by immune cell-mediated antibody-dependent cellular cytotoxicity (ADCC). Notably, human immunoglobulin G (IgG) carries a single paired N-glycan in its constant regions, and the core fucose in these N-glycans negatively regulates ADCC (8). In this regard, antibody therapies without core fucose have been developed and are already clinically used. Core fucosylation is also required for the cell surface expression of programmed cell death 1 (PD-1), and, therefore, the blockade of FUT8 is expected to suppress PD-1 exposure on the cell surface and enhance the potential of cancer immunotherapy (9). In addition, our research group recently generated an anti-core fucosylated human IgG antibody and found that the core fucosylation level of IgG was decreased in the sera of patients with lung cancer. In other words, the level of IgG without core fucose (acore fucosylated IgG) was increased in these patients (10). As described here, core fucose would be a valuable target for the cancer therapy and in the development of cancer biomarkers in the future. Experts agree that probably no protein antigen is truly cancer-specific. However, what would happen if we coordinated them with glycans? It is hoped that the functions of core fucose in cancer will be further elucidated in the near future, and that such a possibility could be studied.
Yuki Ohkawa
| |||||||||||||||||||||||
| References | |
|---|---|
| (1) | Uozumi N, Yanagidani S, Miyoshi E, Ihara Y, Sakuma T, Gao CX, Teshima T, Fujii S, Shiba T, Taniguchi N: Purification and cDNA cloning of porcine brain GDP-L-Fuc:N-acetyl-beta-D-glucosaminide alpha1-->6fucosyltransferase. J. Biol. Chem. 271, 27810-27817, 1996 |
| (2) | Yanagidani S, Uozumi N, Ihara Y, Miyoshi E, Yamaguchi N, Taniguchi N: Purification and cDNA cloning of GDP-L-Fuc:N-acetyl-beta-D-glucosaminide:alpha1-6 fucosyltransferase (alpha1-6 FucT) from human gastric cancer MKN45 cells. J. Biochem. 121, 626-632, 1997 |
| (3) | Zhao Y, Itoh S, Wang X, Isaji T, Miyoshi E, Kariya Y, Miyazaki K, Kawasaki N, Taniguchi N, Gu J: Deletion of core fucosylation on alpha3beta1 integrin down-regulates its functions. J. Biol. Chem. 281, 38343-38350, 2006 |
| (4) | Osumi D, Takahashi M, Miyoshi E, Yokoe S, Lee SH, Noda K, Nakamori S, Gu J, Ikeda Y, Kuroki Y, Sengoku K, Ishikawa M, Taniguchi N: Core fucosylation of E-cadherin enhances cell-cell adhesion in human colon carcinoma WiDr cells. Cancer Sci. 100, 888-895, 2009 |
| (5) | Wang X, Inoue S, Gu J, Miyoshi E, Noda K, Li W, Mizuno-Horikawa Y, Nakano M, Asahi M, Takahashi M, Uozumi N, Ihara S, Lee SH, Ikeda Y, Yamaguchi Y, Aze Y, Tomiyama Y, Fujii J, Suzuki K, Kondo A, Shapiro SD, Lopez-Otin C, Kuwaki T, Okabe M, Honke K, Taniguchi N: Dysregulation of TGF-beta1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proc. Natl. Acad. Sci. U S A 102, 15791-15796, 2005 |
| (6) | Wang X, Gu J, Ihara H, Miyoshi E, Honke K, Taniguchi N: Core fucosylation regulates epidermal growth factor receptor-mediated intracellular signaling. J. Biol. Chem. 281, 2572-2577, 2006 |
| (7) | Bastian K, Scott E, Elliott DJ, Munkley J: FUT8 Alpha-(1,6)-Fucosyltransferase in Cancer. Int. J. Mol. Sci. 22, 455, 2021 |
| (8) | Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M, Hanai N, Shitara K: The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 278, 3466-3473, 2003 |
| (9) | Okada M, Chikuma S, Kondo T, Hibino S, Machiyama H, Yokosuka T, Nakano M, Yoshimura A: Blockage of Core Fucosylation Reduces Cell-Surface Expression of PD-1 and Promotes Anti-tumor Immune Responses of T Cells. Cell Rep. 20, 1017-1028, 2017 |
| (10) | Kanto N, Ohkawa Y, Kitano M, Maeda K, Shiida M, Ono T, Ota F, Kizuka Y, Kunimasa K, Nishino K, Mukai M, Seike M, Azuma A, Harada Y, Fukuda T, Gu J, Taniguchi N: A highly specific antibody against the core fucose of the N-glycan in IgG identifies the pulmonary diseases and its regulation by CCL2. J. Biol. Chem. 299, 105365, 2023 |
Mar. 15, 2024