|
Recent Advances in Structural Glycobiology
|
 |
|
 |
Rapid progress in structural biology has great influence
on glycobiology. The crystal structure of a galactosyltransferase in complex
with sugar-nucleotide and an acceptor analogue reported in 2001 has provided
the structural basis of the formation of glycosidic bonds. Three-dimensional
structures of glycoside hydrases such as  -mannosidase
have been reported one after another, some of which have been classified
into a novel fold. Knowledge on structural basis of interactions between
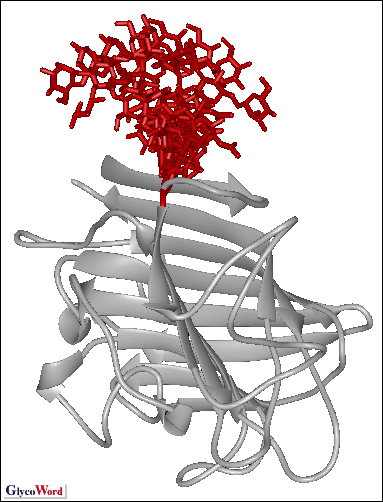
proteins and glycoconjugates has expanded: the crystal structure of human
P-selectin complexed with a cognate glycopeptide visualizes a mode of
binding of the selectin to the glycan as well as to the polypeptide containing
sulfated tyrosine residues. Crystal structures of MHC class I molecules
presenting glycopeptide and CD1 molecules presenting glycolipids have
also been reported recently. Inspection of these data underlines the importance
of structural studies not only of liberated glycans but also of those
conjugated with intrinsic carriers (lipids or polypeptides). -mannosidase
have been reported one after another, some of which have been classified
into a novel fold. Knowledge on structural basis of interactions between
proteins and glycoconjugates has expanded: the crystal structure of human
P-selectin complexed with a cognate glycopeptide visualizes a mode of
binding of the selectin to the glycan as well as to the polypeptide containing
sulfated tyrosine residues. Crystal structures of MHC class I molecules
presenting glycopeptide and CD1 molecules presenting glycolipids have
also been reported recently. Inspection of these data underlines the importance
of structural studies not only of liberated glycans but also of those
conjugated with intrinsic carriers (lipids or polypeptides).
Although the biological importance of glycans expressed on proteins has
been widely recognized, little is known about their specific roles from
the structural aspect. This deficiency in our knowledge is largely due
to the lack of an appropriate methodology to deal with glycoproteins as
targets of structural biology. Carbohydrate moieties exhibit microheterogeneities
and possess a significant degree of freedom in internal motion, which
hampers crystallization or interpretation of electron density. Hence,
X-ray crystallographic analyses of glycoproteins have so far been carried
out with deglycosylated glycoproteins. Recently, crystallographic studies
using recombinant glycoproteins produced in insect cells have been widely
attempted.
Prior to conformational analyses of glycoproteins, their glycoforms must
be determined in advance. Recent advances in HPLC mapping and mass spectrometric
techniques allow us to determine the covalent structures of glycans of
glycoproteins, which greatly facilitates the development of structural
biology of glycoproteins. Now it is possible to perform NMR analyses of
glycoproteins whose glycans are labeled with stable isotopes by metabolic
labeling via biosynthetic pathways of mammalian cells or by in
vitro enzymatic glycosylation onto isolated glycoproteins. This is
opening up a new way for the elucidation of atomic resolution of the structure,
dynamics, and interaction of glycoproteins in solution.
Structural biology of glycoconjugates is an unexplored field beyond structural
genomics, which is highly thought of at present. It is essential to develop
this field and decode the biological signals expressed by glycans. |
|
|
 |
|
Fig. 1 |
|
|
|
|
|
Koichi Kato
(Graduate School of Pharmaceutical Sciences, Nagoya City University) |
|
|
|
|
|
| References |
(1) |
Persson K, Ly HD, Dieckelmann M, Wakarchuk WW, Withers SG, Strynadka
NC: Crystal structure of the retaining galactosyltransferase LgtC
from Neisseria meningitides in complex with donor and acceptor sugar
analogs. Nature Struct. Biol. 8, 166-175, 2001 |
|
(2) |
Somers WS, Tang J, Shaw GD, Camphausen RT: Insights into the molecular
basis of leukocyte tethering and rolling revealed by structures.
Cell 103, 467-479, 2000 |
|
(3) |
Yamaguchi Y, Kato K: Structural biology
of glycoproteins of immunological interest, SEIKAGAKU
74, 43-46, 2002 |
|
|
|
|
|
| Oct. 31, 2002 |
|
|
|
|
|
|
|



