
Tetsuro Ujihara
Technical Research Laboratories, Kyowa Hakko Bio Co., Ltd.
2006, Master of Philosophy, Graduate School of Arts and Sciences, University of Tokyo
2006, Research Scientist, Kyowa Hakko Kogyo Co., Ltd.
2015, Doctor of Philosophy, Graduate School of Arts and Sciences, University of Tokyo
2019–present, Senior Research Scientist, Kyowa Hakko Bio Co., Ltd.
I am interested in the microbial production of valuable products including human milk oligosaccharides. In particular, I am trying to construct useful strains and find processes for efficient large-scale cultivation.

Kazunari Fukumoto
Technical Research Laboratories, Kyowa Hakko Bio Co., Ltd.
2006, Master of Philosophy, Graduate School of Arts and Sciences, University of Tokyo
2006, Research Scientist, Kyowa Hakko Kogyo Co., Ltd.
2018–present, Senior Research Scientist, Kyowa Hakko Bio Co., Ltd.
I am interested in the mass production of useful substances using microorganisms, including human milk oligosaccharides, and am working on the development of production processes mainly in terms of purification.
Human milk oligosaccharides (HMOs) are a diverse group of more than 250 compounds found in human milk and are the third most abundant solid component in breast milk, after lactose and lipids. Until relatively recently, the functions of these HMOs were not well understood because of the difficulty of obtaining them at high quantity and purity and at reasonable cost1). However, technological innovations have so far produced 2'-fucosyllactose (2'-FL), 6'-sialyllactose (6'-SL), and 3'-sialyllactose, and their important physiological functions have been revealed. Such functions include the prevention of infection by pathogens and viruses, improvement of intestinal barrier function, immunomodulatory effects such as anti-inflammatory properties, modulation of intestinal bacterial growth as exemplified by promotion of the growth of beneficial intestinal bacteria such as bifidobacteria, and facilitation of brain development1,2,3. Historically, the preparation of HMOs was carried out by extraction from milk, enzymatic synthesis, and whole-cell reaction, but currently, the direct fermentation method is widely used for manufacturing HMOs (Figure 1). This paper reviews and outlines the history of technological development and the future prospects of practical HMO production.
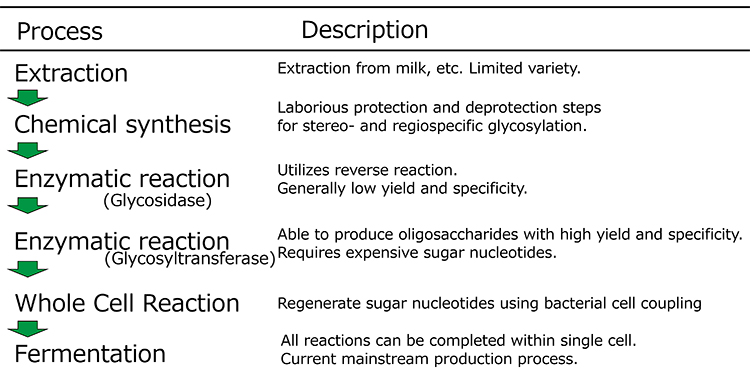
HMOs have traditionally been purified from breast milk to determine their structures. The Snow Brand group, a Japanese company, found that some of the milk oligosaccharides that share the same structure as HMOs can be purified from lactose mother liquor, a byproduct formed in the process of lactose production from whey4. However, those bovine milk oligosaccharides are limited in both variety and quantity, so their extraction from the source is not a realistic process for commercial use. HMOs are composed of more than 250 different structures, and the basic backbone consists of lactose, a β1-4 bond between galactose and glucose, which is elongated by galactose, N-acetylglucosamine (GlcNAc), fucose, and N-acetylneuraminic acid, with various positions and glycosidic linkages1. Because of the presence of many hydroxyl groups in sugars, regiospecific synthesis of sugars is highly challenging, which has hampered their industrial production.
Chemical and enzymatic syntheses have been considered for industrial applications. However, such methods have only been validated for gram-scale production at the laboratory level. They cannot achieve large-scale production on the kilogram scale or larger.
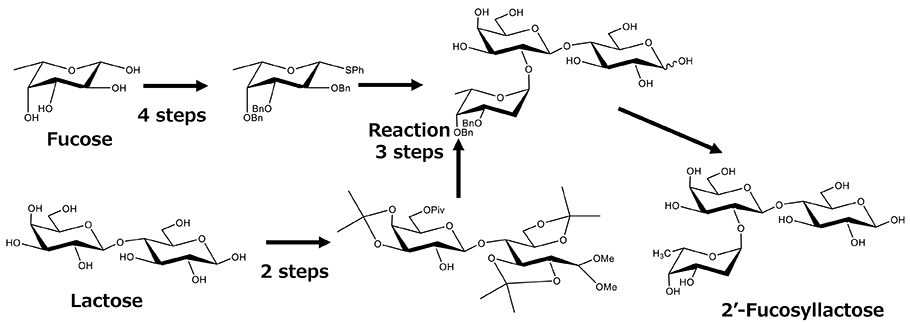
In the chemical synthesis method, the hydroxyl groups of sugars need to be modified with protecting groups to accomplish regiospecific glycosidic bond formation, which should then be deprotected after the synthesis. The protection and deprotection and the related purification steps thus complicate the process. Glycom A/S succeeded in producing 2'-FL and lacto--N-neotetraose (LNnT) by chemical synthesis and used them in preclinical and clinical studies between 2005 and 20125. The method for 2'-FL synthesis involves 1-S-phenylfucose as a donor and lactose 6'-O-pivaloate ester as an acceptor, which is unique in that it can react with a crude acceptor without crystallization and purification. This technique allows synthesis to be achieved with minimal purification steps (Figure 2).
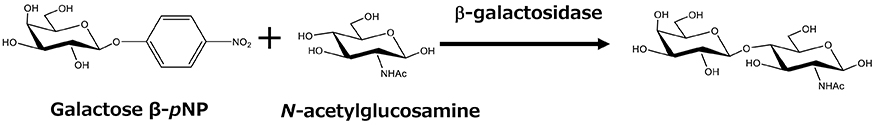
Although progress has been made in chemical synthesis, enzymatic methods are also being considered as a useful tool for HMO synthesis because of their specificity. Several procedures have been developed in which either glycosidases or glycosyltransferases are used. Glycosidase-based methods exploit the reverse hydrolysis reaction of oligosaccharides. Such methods are commonly used in laboratory-scale synthesis because glycosidases are easily obtainable in many cases. Based on the hydrolysis reaction inherently reaching an equilibrium, this method relies on the principle that, if the reaction mixture contains a high concentration of sugars (the products of the hydrolysis), the reverse synthetic reaction proceeds until equilibrium is attained. This method is, however, intrinsically inefficient, and large amounts of substrates remain in the reaction mixture. Ajisaka’s group overcame this drawback by efficiently transferring galactose to GlcNAc by modifying the donor galactose with p-nitrophenol and hydrolyzing it with β-galactosidase in the presence of a large amount of the acceptor GlcNAc6 (Figure 3). Even with this method, the yield was about 50%. In addition, in a study by Nishimoto’s group, lacto-N-biose was synthesized at a yield of 98% using the reverse reaction of phosphorylase7.
In contrast to the method using glycosidases, the production method using glycosyltransferases, which are involved in the biosynthesis of glycan structures in vivo, has the great advantage of being able to synthesize oligosaccharides with the desired structure with high stereospecificity and regiospecificity without producing by-products or introducing any protective groups. However, there were two hurdles to the use of glycosyltransferases. One was that the enzymes were not as readily available as glycosidases. The other was that they require high-energy compounds called sugar nucleotides as substrates (Table 1). Regarding the availability of glycosyltransferases, several mammalian glycosyltransferases with various specificities have been reported previously. However, because they are enzymes from higher eukaryotes, it was until recently quite difficult to express glycosyltransferases recombinantly in E. coli. In the 2000s, it was gradually revealed that bacterial cell surface glycans also have oligosaccharide structures similar to those of mammalian glycans, which indicates that bacteria also serve as a source of glycosyltransferases and that they could be expressed in E. coli. Bacterial glycosyltransferases suitable for expression in E. coli have thus attracted significant attention. With the development of efficient activity-screening methods and rapid progress in bacterial genomic sequence analysis, candidate glycosyltransferase genes have been found in many pathogens, and there have been reports of the isolation and characterization of those genes from Rockefeller University in the United States and research institutes in Canada. Many of these were expressed as active soluble proteins in E. coli. Representative examples are as follows: galactosyltransferase, N-acetylglucosaminyltransferase8, and sialyltransferase9 were obtained from Neisseria meningitidis, a meningococcal pathogen; sialyltransferase was obtained from Campylobacter jejuni, a representative bacterium associated with food poisoning10; and fucosyltransferase was obtained from Helicobacter pylori and others11. Various glycosyltransferase genes found in numerous bacteria have now been registered in a database12.
Table 1. Glycosyltransferases and Sugar nucleotides
| Glycosyltransferase | Sugar nucleotide |
|---|---|
| Glucosyltransferase | UDP-Glc |
| Galactosyltransferase | UDP-Gal |
| N-acetylglucosaminyltransferase | UDP-GlcNAc |
| N-acetylgalactosaminyltransferase | UDP-GalNAc |
| Mannnosyltransferase | GDP-Man |
| Fucosyltransferase | GDP-Fuc |
| Sialyltransferase | CMP-NeuAc |
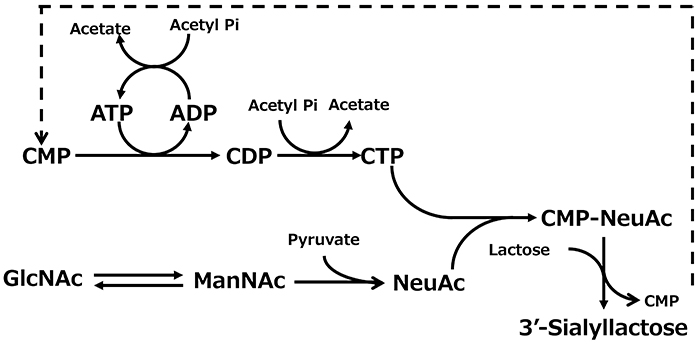
Given that candidate glycosyltransferases are expressed in E. coli, the second problem remains: the need for sugar nucleotides. Sugar nucleotides are generally very expensive, and their low stability has been a challenge for oligosaccharide production using glycosyltransferases. GeneChem, a South Korean company, succeeded in synthesizing the sugar-nucleotide CMP-sialic acid (CMP-NeuAc), which is necessary to produce 3'-SL, from relatively inexpensive N-acetylglucosamine and cytidine monophosphate (CMP)13 (Figure 4). In this process, CMP formed through the transferase reaction is efficiently regenerated into CTP in a recycling system using acetyl phosphate. Another group generated a chimeric protein by fusing CMP-sialic acid synthase and α2,3-sialyltransferase and, using the enzyme, they showed that 3'-SL can be synthesized on a 100-g scale14. Thus, mass synthesis of oligosaccharides using glycosyltransferases had become possible, but application of these methods for the industrial synthesis of oligosaccharides was still limited because they required separate processing conditions for enzyme preparation and sugar-nucleotide regeneration.
The whole-cell reaction method for oligosaccharide production was developed to make the supply of sugar nucleotides easier and more efficient. This method uses the bacterial cell itself as a catalyst, instead of purified enzymes, and thus is simple to perform because purification of enzymes is not required. The reaction is carried out using bacterial cells treated with surfactants or organic solvents, which makes the cell membranes permeable to substances. Consequently, complex multi-step reactions can be accomplished in one pot by simply combining bacterial cells with desired functions. In the enzymatic reaction system described above, ATP should be supplied exogenously or recycled in the presence of acetyl phosphate for regenerating phosphorylated nucleotides, but the whole-cell system is characterized by the fact that ATP can be metabolically generated from an inexpensive sugar source such as glucose. For example, a UDP-Gal production system that uses galactose and orotate, an inexpensive source of nucleic acids, as substrates was constructed using recombinant E. coli cells expressing enzymes involved in the biosynthesis of UDP-galactose (UDP-Gal) and Corynebacterium ammoniagenes cells, which have a high capacity to produce nucleoside 5'-triphosphate. As a result, 44 g/l of UDP-Gal accumulated in 21 h15 (Figure 5). Similarly, upon combining recombinant E. coli cells expressing CMP-sialic acid synthase and CTP synthase with C. ammoniagenes cells, 17 g/l of CMP-NeuAc accumulated in 27 h, starting from orotate and sialic acid16.
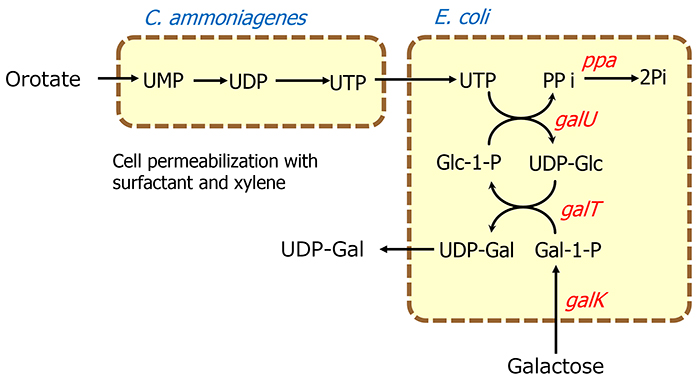
Thus, by combining E. coli cells expressing glycosyltransferases with other cells carrying the sugar nucleotide production system, it was expected that these whole-cell multiple reaction systems could be integrated to create a platform for directly producing oligosaccharides. In fact, by combining E. coli cells expressing the α2,3-sialyltransferase gene from N. gonorrhoeae with cells carrying the CMP-NeuAc production system, 33 g/l of 3'-SL accumulated in 11 h16 (Figure 6). In addition, when E. coli cells expressing the α1,4-galactosyltransferase gene from N. gonorrhoeae were combined with the cells of the UDP-Gal production system, globotriose, an oligosaccharide structure recognized by verotoxin produced by pathogenic E. coli O-157 (Galα1-4Galβ 1-4Glc), accumulated at 188 g/l in 36 h15.
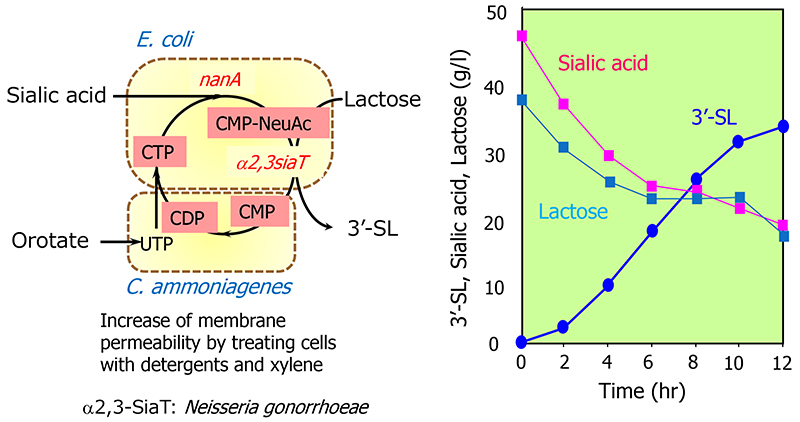
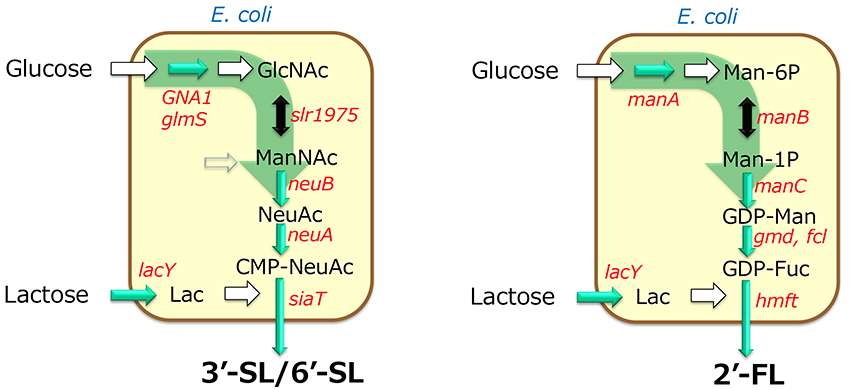
The production of oligosaccharides by the integrated whole-cell bacterial reaction system compensates for the disadvantages of glycosyltransferases, which require expensive and unstable nucleotide sugars as substrates, by using microorganisms to synthesize and regenerate them. This constitutes a breakthrough that enables industrial mass production of oligosaccharides. However, the production method involving multiple cells has the disadvantage of the need for multiple facilities for cell preparation. Therefore, as a new process for oligosaccharide production, a fermentation method was developed in which the synthesis and regeneration of sugar nucleotides, and the expression of glycosyltransferases, are implemented in a single bacterial cell. In this method, the microorganism can synthesize the products during cultivation. Thus, the production facility can be simplified compared with multiple whole-cell reaction processes because the oligosaccharide production can be completed in a single fermenter. For example, in the production of 3'-SL and 6'-SL, the biosynthetic pathway from glucose to N-acetylglucosamine was established by enhancing the activity of glucosamine-6-phosphate synthase (glmS) and introducing the glucosamine-6-phosphate N-acetyltransferase gene (GNA1) from yeast, and by introducing GlcNAc 2-epimerase and sialic acid synthase genes (neuB). As a result, sialic acid can be produced from glucose17. Furthermore, by introducing the genes for CMP-sialic acid synthase and sialyltransferase into this sialic acid-producing bacterium, we successfully established systems for producing 3'-SL and 6'-SL17 (Figure 4). In addition, many microorganisms have been reported to produce 2'-FL by modifying the GDP-fucose biosynthesis system and introducing fucosyltransferase genes (Figure 7).
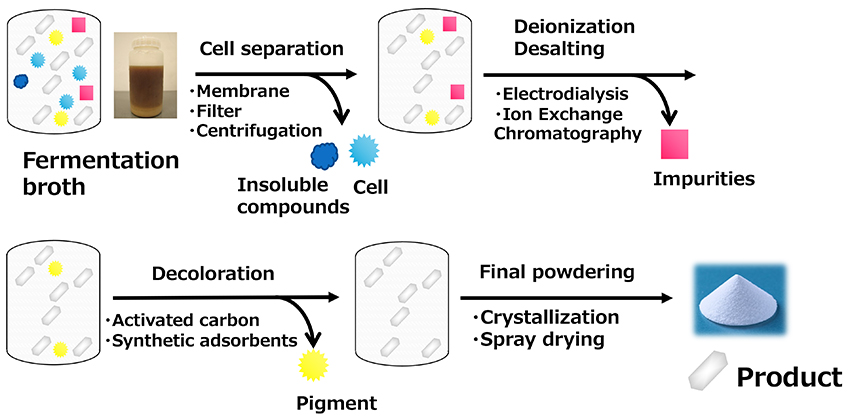
Since the primary use of HMO products is for infant formula supplementation, high purity is required. However, the production methods described so far generate impurities to varying degrees. Therefore, an efficient purification technology is required to purify and powderize them. The HMO synthesized by the fermentation process is present as a dissolved form in the culture medium that contains impurities, such as components derived from the culture medium and bacteria. The bacterial cells are generally removed by a membrane filter and/or centrifugal separation, which is followed by desalting (or deionization) using ion exchange resins or electrodialysis. During the process, charged salts and impurities are separated and removed. The product is further decolorized using activated carbon or synthetic adsorbents to remove pigments and then concentrated. According to the practical application documents submitted by companies, the chromatographic separation process is sometimes included in the process to remove impurities18 (Figure 8). The effectiveness of chromatographic separation depends on the nature of the products. For example, in the case of acidic HMOs such as 3'-SL and 6'-SL, which carry a negative charge, considerable separation can be expected with ion exchange resins, but in the case of neutral HMOs such as 2'-FL and LNnT, only removal of charged compounds can be expected, and methods such as simulated-moving bed chromatography and gel filtration chromatography are required for further separation from impurities, which is a major issue from a productivity perspective19. Crystallization and spray drying are possible methods for the final powdering of the concentrated solution after purification. Crystallization is an effective method of enhancing the purity of neutral HMOs, and was once a focus of attention when companies competed for patent applications, but now many companies use spray drying, which is more productive20. Spray drying is a technology in which liquid raw materials are sprayed into hot air to instantly evaporate water to obtain dried powder.
Following chemical synthesis, enzymatic synthesis, and the whole-cell reaction system, the majority of commercially available HMOs today are produced by the fermentation process. Glycom A/S, for example, initially produced HMOs by chemical synthesis, but is believed to have switched to the fermentation process. Only a few manufacturers, such as GeneChem, produce HMOs by a method other than the fermentation process. 2'-FL, LNnT, 3'-SL, and 6'-SL are the HMO species that are currently produced on a commercial scale. In the future, it is expected that HMOs with more complex structures will be produced by fermentation. For example, a GRAS application to the FDA has been made for pentasaccharide lacto-N-fucopentaose I (LNFPI) as a mixture with 2-FL.21) In this case, the presence of trisaccharides 2'-FL has not caused a problem, but when considering the production of oligosaccharides with a longer chain length, the control of by-product oligosaccharide formation will be an issue. In particular, in the case of the production of uncharged neutral sugars, it is difficult to separate by-product oligosaccharides at the purification stage. In these cases, control at the cultivation stage could be essential. As more HMO species become available in the future, infant formula ingredients will become more similar to those present in breast milk. In this regard, it will become more important for us to elucidate the physiological functions of HMOs.