Role of hyaluronan and proteoglycan link protein | ||||||||||||||||||
 |
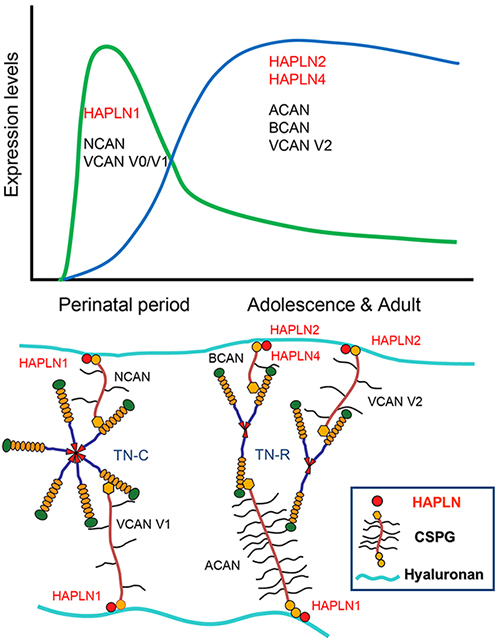
Link protein was discovered as one of the major components of the extracellular matrix (ECM) of abundant cartilage tissue. Biochemical analysis and electron microscopic observation of ECM components isolated from cartilage tissue revealed that a single Link protein molecule binds to a single aggrecan (ACAN) molecule, the major proteoglycan (PG) of cartilage tissue, synergistically stabilizing those non-covalent binding to hyaluronan. The molecular structure of link protein is very similar to the N-terminal G1 domain of the hyaluronan-binding aggrecan family (or lectican family, since it contains a C-type lectin motif in its G3 domain). The B, B' subdomains are approximately 100 amino acid residues long and are also called proteoglycan tandem repeat (PTR) or link modules and are abundant in the link module superfamily with hyaluronan-binding properties. Until the discovery of other family members by molecular cloning techniques, the link protein abundant in cartilage tissue was called cartilage link protein (CRTL1). Three other family members have been cloned after 2000 and they were named the hyaluronan and proteoglycan link protein (HAPLN) gene family (1). Interestingly, all four HAPLN genes are present in the genome in pairs with lectican PG genes such as ACAN. The link module superfamily of hyaluronan-binding genes is a hallmark of vertebrates, and the four paired genes were probably created during evolution.
The perineuronal net (PNN) structure discovered by Golgi and Ramon y Cajar et al. is a mesh-like structure found in the cell bodies and dendrites of certain neurons but is a highly condensed ECM structure on the cell membrane surface. Since HAPLN1 systemically deficient mice are lethal early in life due to chondrodysplasia, analysis of PNN development after birth in mice with rescued expression in cartilage (Crtl1-/-/Crtl1-Tg+/+) showed that PNN accumulation in the cortex was greatly attenuated without significantly affecting the overall expression level of PG (4). Furthermore, the same mice maintained ocular dominance plasticity in the adult. These results indicate that HAPLN1 plays a critical role in the formation of PNNs and the regulation of their plasticity. In contrast, HAPLN4 is expressed predominantly in the cerebellum and brainstem nuclei, where, however, HAPLN1 also co-localizes; in these nuclei of HAPLN4-deficient mice, it affects the localization pattern of the BCAN but not the ACAN. In the deep cerebellar nuclei, our results elucidate HAPLN4 as a PNN component that is selectively responsible for GABAergic Purkinje cells- deep cerebellar nuclei synapse formation (5).
Toshitaka Oohashi
| |||||||||||||||||
| References | |
|---|---|
| (1) | Spicer AP, Joo A, Bowling RA Jr: A hyaluronan binding link protein gene family whose members are physically linked adjacent to chondroitin sulfate proteoglycan core protein genes: the missing links. J. Biol. Chem. 278, 21083-21091, 2003 |
| (2) | Watanabe H, Yamada Y. Mice lacking link protein develop dwarfism and craniofacial abnormalities. Nat. Genet. 21, 225-229, 1999 |
| (3) | Oohashi T, Edamatsu M, Bekku Y, Carulli D: The hyaluronan and proteoglycan link proteins: Organizers of the brain extracellular matrix and key molecules for neuronal function and plasticity. Exp. Neurol. 274, 134-144, 2015 |
| (4) | Carulli D, Pizzorusso T, Kwok JC, Putignano E, Poli A, Forostyak S, Andrews MR, Deepa SS, Glant TT, Fawcett JW: Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain 133, 2331-2347, 2010 |
| (5) | Edamatsu M, Miyano R, Fujikawa A, Fujii F, Hori T, Sakaba T, Oohashi T: Hapln4/Bral2 is a selective regulator for formation and transmission of GABAergic synapses between Purkinje and deep cerebellar nuclei neurons. J. Neurochem. 147, 748-763, 2018 |
| (6) | Bekku Y, Vargová L, Goto Y, Vorísek I, Dmytrenko L, Narasaki M, Ohtsuka A, Fässler R, Ninomiya Y, Syková E, Oohashi T: Bral1: its role in diffusion barrier formation and conduction velocity in the CNS. J. Neurosci. 30, 3113-3123, 2010 |
| (7) | Fawcett JW, Oohashi T, Pizzorusso T: The roles of perineuronal nets and the perinodal extracellular matrixin neuronal function. Nat. Rev. Neurosci. 20, 451-465, 2019 |
Jun 15, 2023